Harvesting prevascularized smooth muscle cell sheets from common polystyrene culture dishes
- PMID: 30256839
- PMCID: PMC6157888
- DOI: 10.1371/journal.pone.0204677
Harvesting prevascularized smooth muscle cell sheets from common polystyrene culture dishes
Abstract
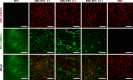
Cell sheet engineering has recently emerged as a promising strategy for scaffold-free tissue engineering. However, the primary method of harvesting cell sheets using temperature-responsive dishes has potential limitations. Here we report a novel cell sheet technology based on a coculture system in which SMCs are cocultured with EPCs on common polystyrene dishes. We found that an intact and highly viable cell sheet could be harvested using mechanical methods when SMCs and EPCs were cocultured on common polystyrene dishes at a ratio of 6:1 for 5 to 6 days; the method is simple, cost-effective and highly repeatable. Moreover, the cocultured cell sheet contained capillary-like networks and could secrete a variety of angiogenic factors. Finally, in vivo studies proved that the cocultured cell sheets were more favorable for the fabrication of vascularized smooth muscle tissues compared to single SMC sheets. This study provides a promising avenue for smooth muscle tissue engineering.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Bladder reconstruction using autologous smooth muscle cell sheets grafted on a pre-vascularized capsule.Theranostics. 2020 Aug 20;10(23):10378-10393. doi: 10.7150/thno.47006. eCollection 2020. Theranostics. 2020. PMID: 32929355 Free PMC article.
-
Layered smooth muscle cell-endothelial progenitor cell sheets derived from the bone marrow augment postinfarction ventricular function.J Thorac Cardiovasc Surg. 2017 Sep;154(3):955-963. doi: 10.1016/j.jtcvs.2017.04.081. Epub 2017 May 24. J Thorac Cardiovasc Surg. 2017. PMID: 28651946 Free PMC article.
-
Bladder reconstruction using scaffold-less autologous smooth muscle cell sheet engineering: early histological outcomes for autoaugmentation cystoplasty.BJU Int. 2014 Dec;114(6):937-45. doi: 10.1111/bju.12685. Epub 2014 Aug 16. BJU Int. 2014. PMID: 25230395
-
Cell sheet-based tissue engineering for fabricating 3-dimensional heart tissues.Circ J. 2014;78(11):2594-603. doi: 10.1253/circj.cj-14-0973. Epub 2014 Oct 16. Circ J. 2014. PMID: 25319318 Review.
-
Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering.J Control Release. 2015 May 10;205:83-8. doi: 10.1016/j.jconrel.2014.12.016. Epub 2014 Dec 16. J Control Release. 2015. PMID: 25523520 Review.
Cited by
-
Engineering pedicled vascularized bladder tissue for functional bladder defect repair.Bioeng Transl Med. 2022 Oct 29;8(5):e10440. doi: 10.1002/btm2.10440. eCollection 2023 Sep. Bioeng Transl Med. 2022. PMID: 37693061 Free PMC article.
-
Measurement of the Adipose Stem Cells Cell Sheets Transmittance.Bioengineering (Basel). 2021 Jul 2;8(7):93. doi: 10.3390/bioengineering8070093. Bioengineering (Basel). 2021. PMID: 34356200 Free PMC article.
-
Bladder reconstruction using autologous smooth muscle cell sheets grafted on a pre-vascularized capsule.Theranostics. 2020 Aug 20;10(23):10378-10393. doi: 10.7150/thno.47006. eCollection 2020. Theranostics. 2020. PMID: 32929355 Free PMC article.
-
The preparation methods and types of cell sheets engineering.Stem Cell Res Ther. 2024 Sep 27;15(1):326. doi: 10.1186/s13287-024-03937-4. Stem Cell Res Ther. 2024. PMID: 39334404 Free PMC article. Review.
-
Recent Progress in in vitro Models for Atherosclerosis Studies.Front Cardiovasc Med. 2022 Jan 27;8:790529. doi: 10.3389/fcvm.2021.790529. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35155603 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

