The Impact of Reactogenicity After the First Dose of Recombinant Zoster Vaccine on the Physical Functioning and Quality of Life of Older Adults: An Open-Label, Phase III Trial
- PMID: 30256905
- PMCID: PMC6625580
- DOI: 10.1093/gerona/gly218
The Impact of Reactogenicity After the First Dose of Recombinant Zoster Vaccine on the Physical Functioning and Quality of Life of Older Adults: An Open-Label, Phase III Trial
Abstract
Background: Herpes zoster and its related complications are associated with significant medical burden, which negatively affects quality of life and daily functioning of the patients. The recently licensed recombinant zoster vaccine (RZV) offers high efficacy but is associated with local and systemic reactions. This study assessed the impact of RZV on the quality of life and daily functioning of participants and implications for caregivers.
Methods: Four hundred and one adults aged 50 years or older received single RZV doses at 0 and 2 months in this open-label, single-arm, multicenter study (NCT02979639). Change in mean SF-36 Physical Functioning score following first-dose administration, quality of life, reactogenicity, safety, productivity loss, and health care resource utilization was assessed. The current analysis was performed post-vaccine dose-1; safety follow-up will continue until 1 year post-dose-2.
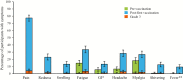
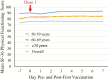
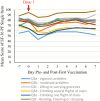
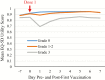
Results: The most common solicited local symptoms were injection-site pain (77.5%), redness (23.0%), and swelling (13.3%); the most frequent solicited systemic reactions were fatigue (33.5%), headache (28.3%), and myalgia (26.8%). Grade 3 reactogenicity occurred in 9.5% of participants and was associated with a transient clinically important decrease in SF-36 Physical Functioning score (affecting activities such as walking, carrying groceries, climbing stairs) on Days 1 and 2 post-first vaccination. No clinically meaningful reductions in mean SF-36 Physical Functioning scale scores from pre- to post-RZV dose-1 were observed (mean +1.9 points, primary end point), and no overall quality-adjusted-life-year loss was recorded post-dose-1. Five participants reported lost workdays; caregiver workload was not increased.
Conclusions: Overall, the physical functioning and quality of life of older adults were not affected by a first RZV dose. The observed reactogenicity was consistent with previous studies.
Keywords: Pain; Physical activity; Physical function; RZV.
© The Author(s) 2018. Published by Oxford University Press on behalf of The Gerontological Society of America.
Figures

Similar articles
-
Impact of Reactogenicity After Two Doses of Recombinant Zoster Vaccine Upon Physical Functioning and Quality of Life: An Open Phase III Trial in Older Adults.J Gerontol A Biol Sci Med Sci. 2021 Feb 25;76(3):485-490. doi: 10.1093/gerona/glaa127. J Gerontol A Biol Sci Med Sci. 2021. PMID: 32530462 Free PMC article. Clinical Trial.
-
Efficacy, reactogenicity, and safety of the adjuvanted recombinant zoster vaccine for the prevention of herpes zoster in Chinese adults ≥ 50 years: A randomized, placebo-controlled trial.Hum Vaccin Immunother. 2024 Dec 31;20(1):2351584. doi: 10.1080/21645515.2024.2351584. Epub 2024 Jun 5. Hum Vaccin Immunother. 2024. PMID: 38838170 Free PMC article. Clinical Trial.
-
A prospective, multi-center post-marketing surveillance cohort study to monitor the safety of the recombinant zoster vaccine in Chinese adults ≥50 years of age.Hum Vaccin Immunother. 2024 Dec 31;20(1):2439031. doi: 10.1080/21645515.2024.2439031. Epub 2024 Dec 16. Hum Vaccin Immunother. 2024. PMID: 39681337 Free PMC article.
-
Safety Profile of the Adjuvanted Recombinant Zoster Vaccine in Immunocompromised Populations: An Overview of Six Trials.Drug Saf. 2021 Jul;44(7):811-823. doi: 10.1007/s40264-021-01076-w. Epub 2021 Jun 11. Drug Saf. 2021. PMID: 34115324 Free PMC article. Review.
-
An Analysis of Spontaneously Reported Data of Vesicular and Bullous Cutaneous Eruptions Occurring Following Vaccination with the Adjuvanted Recombinant Zoster Vaccine.Drug Saf. 2021 Dec;44(12):1341-1353. doi: 10.1007/s40264-021-01118-3. Epub 2021 Oct 7. Drug Saf. 2021. PMID: 34622421 Free PMC article. Review.
Cited by
-
Preclinical immunogenicity of an adenovirus-vectored vaccine for herpes zoster.Hum Vaccin Immunother. 2023 Dec 31;19(1):2175558. doi: 10.1080/21645515.2023.2175558. Epub 2023 Feb 13. Hum Vaccin Immunother. 2023. PMID: 36785938 Free PMC article.
-
Early examination of real-world uptake and second-dose completion of recombinant zoster vaccine in the United States from October 2017 to September 2019.Hum Vaccin Immunother. 2021 Aug 3;17(8):2482-2487. doi: 10.1080/21645515.2021.1879579. Epub 2021 Apr 13. Hum Vaccin Immunother. 2021. PMID: 33849373 Free PMC article.
-
Associations between vaccination and quality of life among Taiwan general population: A comparison between COVID-19 vaccines and flu vaccines.Hum Vaccin Immunother. 2022 Nov 30;18(5):2079344. doi: 10.1080/21645515.2022.2079344. Epub 2022 Jun 9. Hum Vaccin Immunother. 2022. PMID: 35679589 Free PMC article.
-
Factors Influencing Herpes Zoster Vaccine Utilization Among Adults Aged 50 and Above Attending Primary Healthcare Center in Saudi Arabia: A Cross-Sectional Study.Cureus. 2024 Aug 13;16(8):e66761. doi: 10.7759/cureus.66761. eCollection 2024 Aug. Cureus. 2024. PMID: 39280450 Free PMC article.
-
Considering Frailty in SARS-CoV-2 Vaccine Development: How Geriatricians Can Assist.Clin Interv Aging. 2021 Apr 28;16:731-738. doi: 10.2147/CIA.S295522. eCollection 2021. Clin Interv Aging. 2021. PMID: 33953551 Free PMC article. Review.
References
-
- Meyers JL, Madhwani S, Rausch D, Candrilli SD, Krishnarajah G, Yan S. Analysis of real-world health care costs among immunocompetent patients aged 50 years or older with herpes zoster in the United States. Hum Vaccin Immunother. 2017;13:1861–1872. doi:10.1080/21645515.2017.1324373 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

