Signal Detection and Coding in the Accessory Olfactory System
- PMID: 30256909
- PMCID: PMC6211456
- DOI: 10.1093/chemse/bjy061
Signal Detection and Coding in the Accessory Olfactory System
Abstract
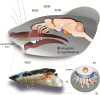
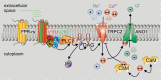
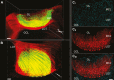
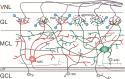
In many mammalian species, the accessory olfactory system plays a central role in guiding behavioral and physiological responses to social and reproductive interactions. Because of its relatively compact structure and its direct access to amygdalar and hypothalamic nuclei, the accessory olfactory pathway provides an ideal system to study sensory control of complex mammalian behavior. During the last several years, many studies employing molecular, behavioral, and physiological approaches have significantly expanded and enhanced our understanding of this system. The purpose of the current review is to integrate older and newer studies to present an updated and comprehensive picture of vomeronasal signaling and coding with an emphasis on early accessory olfactory system processing stages. These include vomeronasal sensory neurons in the vomeronasal organ, and the circuitry of the accessory olfactory bulb. Because the overwhelming majority of studies on accessory olfactory system function employ rodents, this review is largely focused on this phylogenetic order, and on mice in particular. Taken together, the emerging view from both older literature and more recent studies is that the molecular, cellular, and circuit properties of chemosensory signaling along the accessory olfactory pathway are in many ways unique. Yet, it has also become evident that, like the main olfactory system, the accessory olfactory system also has the capacity for adaptive learning, experience, and state-dependent plasticity. In addition to describing what is currently known about accessory olfactory system function and physiology, we highlight what we believe are important gaps in our knowledge, which thus define exciting directions for future investigation.
Figures

Similar articles
-
The rodent accessory olfactory system.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010 Oct;196(10):767-77. doi: 10.1007/s00359-010-0555-z. Epub 2010 Jul 4. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010. PMID: 20607541 Review.
-
Activation of an anatomically distinct subpopulation of accessory olfactory bulb neurons by chemosensory stimulation.Neuroscience. 1999;91(4):1549-56. doi: 10.1016/s0306-4522(98)00711-8. Neuroscience. 1999. PMID: 10391458
-
Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice.Horm Behav. 2004 Sep;46(3):247-56. doi: 10.1016/j.yhbeh.2004.02.009. Horm Behav. 2004. PMID: 15325226 Review.
-
Ex vivo preparations of the intact vomeronasal organ and accessory olfactory bulb.J Vis Exp. 2014 Aug 4;(90):e51813. doi: 10.3791/51813. J Vis Exp. 2014. PMID: 25145699 Free PMC article.
-
MHC molecules in the vomeronasal organ: contributors to pheromonal discrimination?Trends Neurosci. 2003 Dec;26(12):646-50. doi: 10.1016/j.tins.2003.10.001. Trends Neurosci. 2003. PMID: 14624846 Review.
Cited by
-
Neural systems that facilitate the representation of social rank.Philos Trans R Soc Lond B Biol Sci. 2022 Feb 28;377(1845):20200444. doi: 10.1098/rstb.2020.0444. Epub 2022 Jan 10. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 35000438 Free PMC article. Review.
-
The vomeronasal system of the wolf (Canis lupus signatus): The singularities of a wild canid.J Anat. 2024 Jul;245(1):109-136. doi: 10.1111/joa.14024. Epub 2024 Feb 16. J Anat. 2024. PMID: 38366249 Free PMC article.
-
Noradrenaline modulates sensory information in mouse vomeronasal sensory neurons.iScience. 2024 Sep 2;27(10):110872. doi: 10.1016/j.isci.2024.110872. eCollection 2024 Oct 18. iScience. 2024. PMID: 39328934 Free PMC article.
-
Paradoxically Sparse Chemosensory Tuning in Broadly Integrating External Granule Cells in the Mouse Accessory Olfactory Bulb.J Neurosci. 2020 Jul 1;40(27):5247-5263. doi: 10.1523/JNEUROSCI.2238-19.2020. Epub 2020 Jun 5. J Neurosci. 2020. PMID: 32503886 Free PMC article.
-
A revised conceptual framework for mouse vomeronasal pumping and stimulus sampling.Curr Biol. 2024 Mar 25;34(6):1206-1221.e6. doi: 10.1016/j.cub.2024.01.036. Epub 2024 Feb 5. Curr Biol. 2024. PMID: 38320553 Free PMC article.
References
-
- Abdus-Saboor I, Al Nufal MJ, Agha MV, Ruinart de Brimont M, Fleischmann A, Shykind BM.. 2016. An expression refinement process ensures singular odorant receptor gene choice. Curr Biol. 26:1083–1090. - PubMed
-
- Albone ES. 1984. Mammalian semiochemistry : the investigation of chemical signals between mammals. Chichester: John Wiley & Sons, Inc.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

