Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers
- PMID: 30256977
- PMCID: PMC6435099
- DOI: 10.1210/jc.2018-01478
Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers
Abstract
Context: BRAFV600E mutant thyroid cancers are often refractory to radioiodine (RAI).
Objectives: To investigate the utility and molecular underpinnings of enhancing lesional iodide uptake with the BRAF inhibitor vemurafenib in patients with RAI-refractory (RAIR).
Design: This was a pilot trial that enrolled from June 2014 to January 2016.
Setting: Academic cancer center.
Patients: Patients with RAIR, BRAF mutant thyroid cancer.
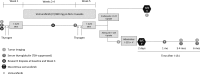
Intervention: Patients underwent thyrotropin-stimulated iodine-124 (124I) positron emission tomography scans before and after ~4 weeks of vemurafenib. Those with increased RAI concentration exceeding a predefined lesional dosimetry threshold (124I responders) were treated with iodine-131 (131I). Response was evaluated with imaging and serum thyroglobulin. Three patients underwent research biopsies to evaluate the impact of vemurafenib on mitogen-activated protein kinase (MAPK) signaling and thyroid differentiation.
Main outcome measure: The proportion of patients in whom vemurafenib increased RAI incorporation to warrant 131I.
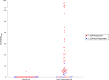
Results: Twelve BRAF mutant patients were enrolled; 10 were evaluable. Four patients were 124I responders on vemurafenib and treated with 131I, resulting in tumor regressions at 6 months. Analysis of research tumor biopsies demonstrated that vemurafenib inhibition of the MAPK pathway was associated with increased thyroid gene expression and RAI uptake. The mean pretreatment serum thyroglobulin value was higher among 124I responders than among nonresponders (30.6 vs 1.0 ng/mL; P = 0.0048).
Conclusions: Vemurafenib restores RAI uptake and efficacy in a subset of BRAF mutant RAIR patients, probably by upregulating thyroid-specific gene expression via MAPK pathway inhibition. Higher baseline thyroglobulin values among responders suggest that tumor differentiation status may be a predictor of vemurafenib benefit.
Trial registration: ClinicalTrials.gov NCT02145143.
Copyright © 2019 Endocrine Society.
Figures



Comment in
-
Still Perfecting Radioiodine in Thyroid Cancer, After All These Years.J Clin Endocrinol Metab. 2019 May 1;104(5):1655-1657. doi: 10.1210/jc.2018-02437. J Clin Endocrinol Metab. 2019. PMID: 30462299 No abstract available.
References
-
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION investigators . Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. - PMC - PubMed
-
- Cabanillas ME, Schlumberger M, Jarzab B, Martins RG, Pacini F, Robinson B, McCaffrey JC, Shah MH, Bodenner DL, Topliss D, Andresen C, O’Brien JP, Ren M, Funahashi Y, Allison R, Elisei R, Newbold K, Licitra LF, Sherman SI, Ball DW. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: a clinical outcomes and biomarker assessment. Cancer. 2015;121(16):2749–2756. - PMC - PubMed
-
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

