Multiple-point magnetic resonance acoustic radiation force imaging
- PMID: 30257059
- PMCID: PMC6642829
- DOI: 10.1002/mrm.27477
Multiple-point magnetic resonance acoustic radiation force imaging
Abstract
Purpose: To implement and evaluate an efficient multiple-point MR acoustic radiation force imaging pulse sequence that can volumetrically measure tissue displacement and evaluate tissue stiffness using focused ultrasound (FUS) radiation force.
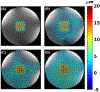
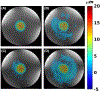
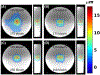
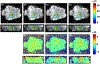
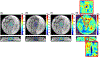
Methods: Bipolar motion-encoding gradients were added to a gradient-recalled echo segmented EPI pulse sequence with both 2D and 3D acquisition modes. Multiple FUS-ON images (FUS power > 0 W) were interleaved with a single FUS-OFF image (FUS power = 0 W) on the TR level, enabling simultaneous measurements of volumetric tissue displacement (by complex subtraction of the FUS-OFF image from the FUS-ON images) and proton resonance frequency shift MR thermometry (from the OFF image). Efficiency improvements included partial Fourier acquisition, parallel imaging, and encoding up to 4 different displacement positions into a single image. Experiments were performed in homogenous and dual-stiffness phantoms, and in ex vivo porcine brain.
Results: In phantoms, 16-point multiple-point magnetic resonance acoustic radiation force imaging maps could be acquired in 5 s to 10 s for a 2D slice, and 60 s for a 3D volume, using parallel imaging and encoding 2 displacement positions/image. In ex vivo porcine brain, 16-point multiple-point magnetic resonance acoustic radiation force imaging maps could be acquired in 20 s for a 3D volume, using partial Fourier and parallel imaging and encoding 4 displacement positions/image. In 1 experiment it was observed that tissue displacement in ex vivo brain decreased by approximately 22% following FUS ablation.
Conclusion: With the described efficiency improvements it is possible to acquire volumetric multiple-point magnetic resonance acoustic radiation force imaging maps, with simultaneous proton resonance frequency shift MR thermometry maps, in clinically acceptable times.
Keywords: ARFI; FUS; HIFU; acoustic radiation force imaging.
© 2018 International Society for Magnetic Resonance in Medicine.
Figures


Similar articles
-
Simultaneous MR thermometry and acoustic radiation force imaging using interleaved acquisition.Magn Reson Med. 2018 Mar;79(3):1515-1524. doi: 10.1002/mrm.26827. Epub 2017 Aug 10. Magn Reson Med. 2018. PMID: 28795419 Free PMC article.
-
Ultrasound focusing using magnetic resonance acoustic radiation force imaging: application to ultrasound transcranial therapy.Med Phys. 2010 Jun;37(6):2934-42. doi: 10.1118/1.3395553. Med Phys. 2010. PMID: 20632605
-
Evaluation of a three-dimensional MR acoustic radiation force imaging pulse sequence using a novel unbalanced bipolar motion encoding gradient.Magn Reson Med. 2016 Sep;76(3):803-13. doi: 10.1002/mrm.25971. Epub 2015 Oct 7. Magn Reson Med. 2016. PMID: 26445135 Free PMC article.
-
Magnetic Resonance Acoustic Radiation Force Imaging (MR-ARFI).J Magn Reson Imaging. 2025 Jul;62(1):20-39. doi: 10.1002/jmri.29712. Epub 2025 Jan 22. J Magn Reson Imaging. 2025. PMID: 39842847 Free PMC article. Review.
-
MR techniques for guiding high-intensity focused ultrasound (HIFU) treatments.J Magn Reson Imaging. 2018 Feb;47(2):316-331. doi: 10.1002/jmri.25770. Epub 2017 Jun 5. J Magn Reson Imaging. 2018. PMID: 28580706 Review.
Cited by
-
The value of contrast-enhanced ultrasound versus shear wave elastography in differentiating benign and malignant superficial lymph node lesions.Am J Transl Res. 2021 Oct 15;13(10):11625-11631. eCollection 2021. Am J Transl Res. 2021. PMID: 34786088 Free PMC article.
-
Simultaneous proton resonance frequency T1 - MR shear wave elastography for MR-guided focused ultrasound multiparametric treatment monitoring.Magn Reson Med. 2023 Jun;89(6):2171-2185. doi: 10.1002/mrm.29587. Epub 2023 Jan 19. Magn Reson Med. 2023. PMID: 36656135 Free PMC article.
-
Magnetic resonance shear wave elastography using transient acoustic radiation force excitations and sinusoidal displacement encoding.Phys Med Biol. 2021 Feb 26;66(5):10.1088/1361-6560/abd5ce. doi: 10.1088/1361-6560/abd5ce. Phys Med Biol. 2021. PMID: 33352538 Free PMC article.
-
Reduced-field of view three-dimensional MR acoustic radiation force imaging with a low-rank reconstruction for targeting transcranial focused ultrasound.Magn Reson Med. 2022 Dec;88(6):2419-2431. doi: 10.1002/mrm.29403. Epub 2022 Aug 2. Magn Reson Med. 2022. PMID: 35916311 Free PMC article.
-
Efficient shear wave elastography using transient acoustic radiation force excitations and MR displacement encoding.Magn Reson Med. 2019 May;81(5):3153-3167. doi: 10.1002/mrm.27647. Epub 2019 Jan 21. Magn Reson Med. 2019. PMID: 30663806 Free PMC article.
References
-
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005;5:321–327. - PubMed
-
- Hectors S, Jacobs I, Moonen C, Strijkers GJ, Nicolay K. MRI methods for the evaluation of high intensity focused ultrasound tumor treatment: current status and future needs. Magn Reson Med 2016;75:302–317. - PubMed
-
- Napoli A, Anzidei M, Marincola BC, et al. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol 2013;48:351–358. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

