Design of a True Bivalent Ligand with Picomolar Binding Affinity for a G Protein-Coupled Receptor Homodimer
- PMID: 30257092
- PMCID: PMC9366271
- DOI: 10.1021/acs.jmedchem.8b01249
Design of a True Bivalent Ligand with Picomolar Binding Affinity for a G Protein-Coupled Receptor Homodimer
Abstract
Bivalent ligands have emerged as chemical tools to study G protein-coupled receptor dimers. Using a combination of computational, chemical, and biochemical tools, here we describe the design of bivalent ligand 13 with high affinity ( KDB1 = 21 pM) for the dopamine D2 receptor (D2R) homodimer. Bivalent ligand 13 enhances the binding affinity relative to monovalent compound 15 by 37-fold, indicating simultaneous binding at both protomers. Using synthetic peptides with amino acid sequences of transmembrane (TM) domains of D2R, we provide evidence that TM6 forms the interface of the homodimer. Notably, the disturber peptide TAT-TM6 decreased the binding of bivalent ligand 13 by 52-fold and had no effect on monovalent compound 15, confirming the D2R homodimer through TM6 ex vivo. In conclusion, by using a versatile multivalent chemical platform, we have developed a precise strategy to generate a true bivalent ligand that simultaneously targets both orthosteric sites of the D2R homodimer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




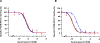

References
-
- Farran B. An update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol. Res 2017, 117, 303–327. - PubMed
-
- Santos R; Ursu O; Gaulton A; Bento AP; Donadi RS; Bologa CG; Karlsson A; Al-Lazikani B; Hersey A; Oprea TI; Overington JP A comprehensive map of molecular drug targets. Nat. Rev. Drug Discovery 2017, 16, 19–34. - PMC - PubMed
- Gaitonde SA; González-Maeso J. Contribution of heteromerization to G protein-coupled receptor function. Curr. Opin. Pharmacol 2017, 32, 23–31. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

