Antioxidant and Fluorescence Properties of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO₄2-/ZrO₂ Solid Superacids Catalyst
- PMID: 30257413
- PMCID: PMC6222355
- DOI: 10.3390/molecules23102445
Antioxidant and Fluorescence Properties of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO₄2-/ZrO₂ Solid Superacids Catalyst
Abstract
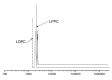
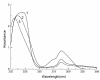
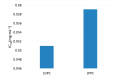
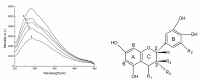
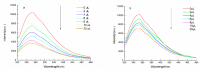
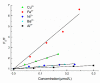
Larix bark oligomeric proanthocyanidins (LOPC) were prepared from larix bark polymeric proanthocyanidins (LPPC) by catalytic hydrogenolysis using SO₄2-/ZrO₂ solid superacid as the catalyst. The catalyst to polymeric proanthocyanidins ratio was 0.2:1 (m/m). The LOPC, obtained after hydrogenolysis at 100 °C for 4 h under 3 MPa hydrogen pressure, retained the structural characteristics of proanthocyanidins. The average degree of polymerization was reduced from 9.50% to 4.76% and the depolymerization yield was 53.85%. LOPC has good antioxidant properties and, at the same concentration, the reducing ability of LOPC was much higher than that of LPPC. The IC50 values of LOPC for scavenging DPPH• and ABTS•+ radicals were 0.046 mg/mL and 0.051 mg/mL, respectively. LOPC is biocompatible and has fluorescent properties that are affected by external factors, such as solvent polarity, pH and the presence of different metal ions. These features indicate that LOPC could be developed as a new biological fluorescent marker. The depolymerization of low-value polymeric proanthocyanidins to provide high-value oligomeric proanthocyanidins and the development of new applications for proanthocyanidins represent significant advances.
Keywords: SO42−/ZrO2 solid superacid; antioxidant activity; catalytic hydrogenolysis; fluorescence; proanthocyanidins.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
Figures







References
-
- Porter L.J., Newman R.H., Foo L.Y., Wong H., Hemingway R.W. Polymeric proanthocyanidins. 13 C NMR studies of procyanidins. J. Chem. Soc. Perkin Trans. 1982;1:1217–1221. doi: 10.1039/p19820001217. - DOI
-
- Pekić B., Kovač V., Alonso E., Revilla E. Study of the extraction of proanthocyanidins from grape seeds. Food Chem. 1998;61:201–206. doi: 10.1016/S0308-8146(97)00128-3. - DOI
-
- Hemingway R.W., Karchesy J.J. Chemistry and Significance of Condensed Tannins. Springer Science & Business Media; Berlin, Germany: 2012.
-
- Du X., Lu Z., Tao Y., Liao X., Shi B. Study on Antioxidant Activity of Catalytic Hydrogenation Products of Larch Polyproanthocyanidins. J. Sichuan Univ. 2005;6:68–73.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

