Optimized Expression and Characterization of a Novel Fully Human Bispecific Single-Chain Diabody Targeting Vascular Endothelial Growth Factor165 and Programmed Death-1 in Pichia pastoris and Evaluation of Antitumor Activity In Vivo
- PMID: 30257416
- PMCID: PMC6213929
- DOI: 10.3390/ijms19102900
Optimized Expression and Characterization of a Novel Fully Human Bispecific Single-Chain Diabody Targeting Vascular Endothelial Growth Factor165 and Programmed Death-1 in Pichia pastoris and Evaluation of Antitumor Activity In Vivo
Abstract
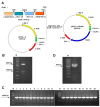
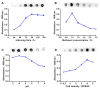
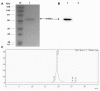
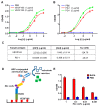
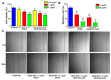
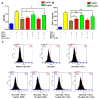
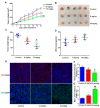
Bispecific antibodies, which can bind to two different epitopes on the same or different antigens simultaneously, have recently emerged as attractive candidates for study in various diseases. Our present study successfully constructs and expresses a fully human, bispecific, single-chain diabody (BsDb) that can bind to vascular endothelial growth factor 165 (VEGF165) and programmed death-1 (PD-1) in Pichia pastoris. Under the optimal expression conditions (methanol concentration, 1%; pH, 4.0; inoculum density, OD600 = 4, and the induction time, 96 h), the maximum production level of this BsDb is achieved at approximately 20 mg/L. The recombinant BsDb is purified in one step using nickel-nitrilotriacetic acid (Ni-NTA) column chromatography with a purity of more than 95%. Indirect enzyme-linked immune sorbent assay (ELISA) and sandwich ELISA analyses show that purified BsDb can bind specifically to VEGF165 and PD-1 simultaneously with affinities of 124.78 nM and 25.07 nM, respectively. Additionally, the BsDb not only effectively inhibits VEGF165-stimulated proliferation, migration, and tube formation in primary human umbilical vein endothelial cells (HUVECs), but also significantly improves proliferation and INF-γ production of activated T cells by blocking PD-1/PD-L1 co-stimulation. Furthermore, the BsDb displays potent antitumor activity in mice bearing HT29 xenograft tumors by inhibiting tumor angiogenesis and activating immune responses in the tumor microenvironment. Based on these results, we have prepared a potential bispecific antibody drug that can co-target both VEGF165 and PD-1 for the first time. This work provides a stable foundation for the development of new strategies by the combination of an angiogenesis inhibition and immune checkpoint blockade for cancer therapy.
Keywords: Pichia pastoris; anti-angiogenesis; bispecific single-chain diabody; immunotherapy; programmed death-1; vascular endothelial growth factor165.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics.MAbs. 2014;6(5):1243-54. doi: 10.4161/mabs.29445. Epub 2014 Oct 30. MAbs. 2014. PMID: 25517309 Free PMC article.
-
Secretory expression of a bispecific antibody targeting tumor necrosis factor and ED-B fibronectin in Pichia pastoris and its functional analysis.Biotechnol Lett. 2014 Dec;36(12):2425-31. doi: 10.1007/s10529-014-1630-2. Epub 2014 Aug 17. Biotechnol Lett. 2014. PMID: 25129049
-
Development of tetravalent IgG1 dual targeting IGF-1R-EGFR antibodies with potent tumor inhibition.Arch Biochem Biophys. 2012 Oct 15;526(2):206-18. doi: 10.1016/j.abb.2012.03.016. Epub 2012 Mar 21. Arch Biochem Biophys. 2012. PMID: 22464987
-
Multispecific Antibodies Targeting PD-1/PD-L1 in Cancer.BioDrugs. 2025 May;39(3):427-444. doi: 10.1007/s40259-025-00712-6. Epub 2025 Mar 19. BioDrugs. 2025. PMID: 40106158 Review.
-
Combined blockade of vascular endothelial growth factor and programmed death 1 pathways in advanced kidney cancer.Clin Adv Hematol Oncol. 2017 Jun;15(6):478-488. Clin Adv Hematol Oncol. 2017. PMID: 28749908 Review.
Cited by
-
The enhanced antitumor activity of bispecific antibody targeting PD-1/PD-L1 signaling.Cell Commun Signal. 2024 Mar 12;22(1):179. doi: 10.1186/s12964-024-01562-5. Cell Commun Signal. 2024. PMID: 38475778 Free PMC article. Review.
-
Developing a dual VEGF/PDL1 inhibitor based on high-affinity scFv heterodimers as an anti-cancer therapeutic strategy.Sci Rep. 2023 Jul 24;13(1):11923. doi: 10.1038/s41598-023-39076-8. Sci Rep. 2023. PMID: 37488176 Free PMC article.
-
Therapeutic targeting of VEGF and/or TGF-β to enhance anti-PD-(L)1 therapy: The evidence from clinical trials.Front Oncol. 2022 Jul 26;12:905520. doi: 10.3389/fonc.2022.905520. eCollection 2022. Front Oncol. 2022. PMID: 35957885 Free PMC article. Review.
-
Recombinant expressing angiopep-2 fused anti-VEGF single chain Fab (scFab) could cross blood-brain barrier and target glioma.AMB Express. 2019 Oct 15;9(1):165. doi: 10.1186/s13568-019-0869-3. AMB Express. 2019. PMID: 31617104 Free PMC article.
-
Identification of a novel fully human anti-toxic shock syndrome toxin (TSST)-1 single-chain variable fragment antibody averting TSST-1-induced mitogenesis and cytokine secretion.BMC Biotechnol. 2022 Oct 28;22(1):31. doi: 10.1186/s12896-022-00760-8. BMC Biotechnol. 2022. PMID: 36307814 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

