Renal Injury during Long-Term Crizotinib Therapy
- PMID: 30257437
- PMCID: PMC6213486
- DOI: 10.3390/ijms19102902
Renal Injury during Long-Term Crizotinib Therapy
Abstract
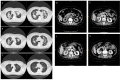
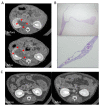
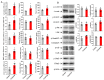
Crizotinib is highly effective against anaplastic lymphoma kinase-positive and c-ros oncogen1-positive non-small cell lung cancer. Renal dysfunction is associated with crizotinib therapy but the mechanism is unknown. Here, we report a case of anaplastic lymphoma kinase positive non-small cell lung cancer showing multiple cysts and dysfunction of the kidneys during crizotinib administration. We also present results demonstrating that long-term crizotinib treatment induces fibrosis and dysfunction of the kidneys by activating the tumor necrosis factor-α/nuclear factor-κB signaling pathway. In conclusion, this study shows the renal detrimental effects of crizotinib, suggesting the need of careful monitoring of renal function during crizotinib therapy.
Keywords: anaplastic lymphoma kinase; crizotinib; cystic formation; fibrosis; lung cancer; renal injury.
Conflict of interest statement
The authors report no declarations of interest regarding data reported in this manuscript.
Figures

References
-
- Christensen J.G., Zou H.Y., Arango M.E., Li Q., Lee J.H., McDonnell S.R., Yamazaki S., Alton G.R., Mroczkowski B., Los G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. - DOI - PubMed
-
- Zou H.Y., Li Q., Lee J.H., Arango M.E., McDonnell S.R., Yamazaki S., Koudriakova T.B., Alton G., Cui J.J., Kung P.P., et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. - DOI - PubMed
-
- Blackhall F.H., Peters S., Bubendorf L., Dafni U., Kerr K.M., Hager H., Soltermann A., O'Byrne K.J., Dooms C., Sejda A., et al. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: Results from the European Thoracic Oncology Platform Lungscape Project. J. Clin. Oncol. 2014;32:2780–2787. doi: 10.1200/JCO.2013.54.5921. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

