Filamentous Aggregates of Tau Proteins Fulfil Standard Amyloid Criteria Provided by the Fuzzy Oil Drop (FOD) Model
- PMID: 30257460
- PMCID: PMC6213535
- DOI: 10.3390/ijms19102910
Filamentous Aggregates of Tau Proteins Fulfil Standard Amyloid Criteria Provided by the Fuzzy Oil Drop (FOD) Model
Abstract
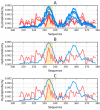
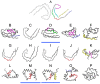
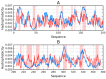
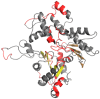
Abnormal filamentous aggregates that are formed by tangled tau protein turn out to be classic amyloid fibrils, meeting all the criteria defined under the fuzzy oil drop model in the context of amyloid characterization. The model recognizes amyloids as linear structures where local hydrophobicity minima and maxima propagate in an alternating manner along the fibril's long axis. This distribution of hydrophobicity differs greatly from the classic monocentric hydrophobic core observed in globular proteins. Rather than becoming a globule, the amyloid instead forms a ribbonlike (or cylindrical) structure.
Keywords: Alzheimer’s disease; tau amyloid; tauopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
The Amyloid as a Ribbon-Like Micelle in Contrast to Spherical Micelles Represented by Globular Proteins.Molecules. 2019 Dec 3;24(23):4395. doi: 10.3390/molecules24234395. Molecules. 2019. PMID: 31816829 Free PMC article. Review.
-
Amyloidogenesis of Tau protein.Protein Sci. 2017 Nov;26(11):2126-2150. doi: 10.1002/pro.3275. Epub 2017 Sep 13. Protein Sci. 2017. PMID: 28833749 Free PMC article. Review.
-
The structure and phase of tau: from monomer to amyloid filament.Cell Mol Life Sci. 2021 Mar;78(5):1873-1886. doi: 10.1007/s00018-020-03681-x. Epub 2020 Oct 19. Cell Mol Life Sci. 2021. PMID: 33078207 Free PMC article. Review.
-
A Peptide Strategy for Inhibiting Different Protein Aggregation Pathways.Chemistry. 2024 Sep 16;30(52):e202400080. doi: 10.1002/chem.202400080. Epub 2024 Sep 3. Chemistry. 2024. PMID: 38972842
-
The twisted tauopathies: surface interactions of helically patterned filaments seen in alzheimer's disease and elsewhere.Soft Matter. 2016 Jan 21;12(3):779-89. doi: 10.1039/c5sm02022k. Epub 2015 Nov 3. Soft Matter. 2016. PMID: 26526630 Free PMC article.
Cited by
-
The Amyloid as a Ribbon-Like Micelle in Contrast to Spherical Micelles Represented by Globular Proteins.Molecules. 2019 Dec 3;24(23):4395. doi: 10.3390/molecules24234395. Molecules. 2019. PMID: 31816829 Free PMC article. Review.
-
Model of Early Stage Intermediate in Respect to Its Final Structure.Biomolecules. 2019 Dec 12;9(12):866. doi: 10.3390/biom9120866. Biomolecules. 2019. PMID: 31842350 Free PMC article.
-
The Structure of Amyloid Versus the Structure of Globular Proteins.Int J Mol Sci. 2020 Jun 30;21(13):4683. doi: 10.3390/ijms21134683. Int J Mol Sci. 2020. PMID: 32630137 Free PMC article.
-
Alternative Structures of α-Synuclein.Molecules. 2020 Jan 30;25(3):600. doi: 10.3390/molecules25030600. Molecules. 2020. PMID: 32019169 Free PMC article.
-
Different Synergy in Amyloids and Biologically Active Forms of Proteins.Int J Mol Sci. 2019 Sep 9;20(18):4436. doi: 10.3390/ijms20184436. Int J Mol Sci. 2019. PMID: 31505841 Free PMC article.
References
-
- Rafii M.S., Lukic A.S., Andrews R.D., Brewer J., Rissman R.A., Strother S.C., Wernick M.N., Pennington C., Mobley W.C., Ness S., et al. PET Imaging of Tau Pathology and Relationship to Amyloid, Longitudinal MRI, and Cognitive Change in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI) J. Alzheimers Dis. 2017;60:439–450. doi: 10.3233/JAD-170390. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

