Galvanic Replacement of Electrochemically Restructured Copper Electrodes with Gold and Its Electrocatalytic Activity for Nitrate Ion Reduction
- PMID: 30257501
- PMCID: PMC6215138
- DOI: 10.3390/nano8100756
Galvanic Replacement of Electrochemically Restructured Copper Electrodes with Gold and Its Electrocatalytic Activity for Nitrate Ion Reduction
Abstract
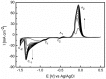
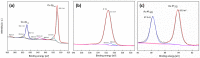
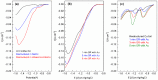
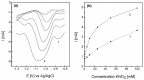
The electrochemical formation of nanostructured materials is a cost effective route to creating substrates that can be employed in a variety of applications. In this work the surface of a copper electrode was electrochemically restructured in an alkaline solution containing ethanol as an additive to modify the surface morphology, and generate a Cu/Cu₂O surface, which is known to be active for the electrocatalytic reduction of environmentally harmful nitrate ions. To increase the activity of the nanostructured surface it was decorated with gold prisms through a facile galvanic replacement approach to create an active Cu/Cu₂O/Au layer. The surface was characterized by scanning electron microscopy, energy dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy, as well as electrochemical techniques. It was found that the presence of recalcitrant oxides, and Au was beneficial for the increased activity compared to unmodified copper and undecorated restructured copper and was consistent with the incipient hydrous oxide adatom mediator model of electrocatalysis. This approach to generating nanostructured metal/metal oxide surfaces that can be galvanically replaced to create these types of composites may have other applications in the area of electrocatalysis.
Keywords: active sites; electrocatalysis; galvanic replacement; hydrous oxides; nanostructures; nitrate reduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sun I.-W., Chang J.-K. Electrodeposition of nanomaterials. In: Breitkopf C., Swider-Lyons K., editors. Springer Handbook of Electrochemical Energy. Springer; Berlin/Heidelberg, Germany: 2017. pp. 835–895.
-
- Davydov A.D., Volgin V.M. Template electrodeposition of metals. Review. Russ. J. Electrochem. 2016;52:806–831. doi: 10.1134/S1023193516090020. - DOI
-
- Braun T.M., Schwartz D.T. The emerging role of electrodeposition in additive manufacturing. Electrochem. Soc. Interface. 2016;25:69–73. doi: 10.1149/2.F07161if. - DOI
-
- Paul S. Nanomaterials synthesis by electrodeposition techniques for high-energetic electrodes in fuel cell. Nanomater. Energy. 2015;4:80–89. doi: 10.1680/nme.14.00031. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

