Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives
- PMID: 30257514
- PMCID: PMC6321143
- DOI: 10.3390/pharmaceutics10040167
Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives
Abstract
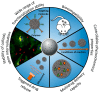
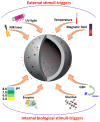
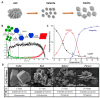
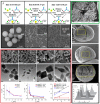
Porous inorganic nanostructured materials are widely used nowadays as drug delivery carriers due to their adventurous features: suitable architecture, large surface area and stability in the biological fluids. Among the different types of inorganic porous materials, silica, calcium carbonate, and calcium phosphate have received significant attention in the last decade. The use of porous inorganic materials as drug carriers for cancer therapy, gene delivery etc. has the potential to improve the life expectancy of the patients affected by the disease. The main goal of this review is to provide general information on the current state of the art of synthesis of the inorganic porous particles based on silica, calcium carbonate and calcium phosphate. Special focus is dedicated to the loading capacity, controllable release of drugs under internal biological stimuli (e.g., pH, redox, enzymes) and external noninvasive stimuli (e.g., light, magnetic field, and ultrasound). Moreover, the diverse compounds to deliver with silica, calcium carbonate and calcium phosphate particles, ranging from the commercial drugs to genetic materials are also discussed.
Keywords: calcium carbonate; calcium phosphate; drug delivery systems; drug loading; in vitro and in vivo delivery; silica-based particles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



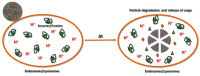
References
-
- Timin A.S., Khashirova S.Y., Zhansitov A., Rumyantsev E.V. Synthesis and application of silica hybrids grafted with new guanidine-containing polymers as highly effective adsorbents for bilirubin removal. Colloid Polym. Sci. 2015;293:1667–1674. doi: 10.1007/s00396-015-3555-2. - DOI
-
- Timin A.S., Rumyantsev E.V., Solomonov A.V., Musabirov I.I., Sergeev S.N., Ivanov S.P., Berlier G., Balantseva E. Preparation and characterization of organo-functionalized silicas for bilirubin removal. Colloids Surf. Physicochem. Eng. Asp. 2015;464:65–77. doi: 10.1016/j.colsurfa.2014.10.012. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

