A mitochondrial based oncology platform for targeting cancer stem cells (CSCs): MITO-ONC-RX
- PMID: 30257595
- PMCID: PMC6226227
- DOI: 10.1080/15384101.2018.1515551
A mitochondrial based oncology platform for targeting cancer stem cells (CSCs): MITO-ONC-RX
Abstract
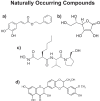
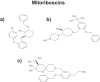
Here, we wish to propose a new systematic approach to cancer therapy, based on the targeting of mitochondrial metabolism, especially in cancer stem cells (CSCs). In the future, we envision that anti-mitochondrial therapy would ultimately be practiced as an add-on to more conventional therapy, largely for the prevention of tumor recurrence and cancer metastasis. This mitochondrial based oncology platform would require a panel of FDA-approved therapeutics (e.g. Doxycycline) that can safely be used to inhibit mitochondrial OXPHOS and/or biogenesis in CSCs. In addition, new therapeutics that target mitochondria could also be developed, to optimize their ability to eradicate CSCs. Finally, in this context, mitochondrial-based biomarkers (i.e. "Mito-signatures") could be utilized as companion diagnostics, to identify high-risk cancer patients at diagnosis, facilitating the early detection of tumor recurrence and the prevention of treatment failure. In summary, we suggest that new clinical trials are warranted to test and possibly implement this emerging treatment strategy, in a variety of human cancer types. This general approach, using FDA-approved antibiotics to target mitochondria, was effective in killing CSCs originating from many different cancer types, including DCIS, breast (ER(+) and ER(-)), prostate, ovarian, lung and pancreatic cancers, as well as melanoma and glioblastoma, among others. Thus, we propose the term MITO-ONC-RX, to describe this anti-mitochondrial platform for targeting CSCs. The use of re-purposed FDA-approved drugs will undoubtedly help to accelerate the clinical evaluation of this approach, as these drugs can move directly into Phase II clinical trials, saving considerable amounts of time (10-15 y) and billions in financial resources.
Keywords: Mito-signatures; Mito-therapeutics; Mitochondria; Mitoflavoscins; Mitoketoscins; Mitoriboscins; drug discovery; mito-biomarkers; oncology platform.
Figures










References
-
- Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, et al. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017. January;14(1):11–31. - PubMed
-
- Aguilar-Gallardo C, Simón C.. Cells, stem cells, and cancer stem cells. Semin Reprod Med. 2013. January;31(1):5–13. - PubMed
-
- Apostoli AJ, Ailles L. Clonal evolution and tumor-initiating cells: new dimensions in cancer patient treatment. Crit Rev Clin Lab Sci. 2016;53(1):40–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
