Innate humoral immune defences in mammals and insects: The same, with differences ?
- PMID: 30257608
- PMCID: PMC7000196
- DOI: 10.1080/21505594.2018.1526531
Innate humoral immune defences in mammals and insects: The same, with differences ?
Abstract
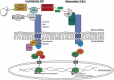
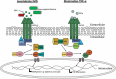
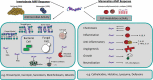
The insect immune response demonstrates many similarities to the innate immune response of mammals and a wide range of insects is now employed to assess the virulence of pathogens and produce results comparable to those obtained using mammals. Many of the humoral responses in insects and mammals are similar (e.g. insect transglutaminases and human clotting factor XIIIa) however a number show distinct differences. For example in mammals, melanization plays a role in protection from solar radiation and in skin and hair pigmentation. In contrast, insect melanization acts as a defence mechanism in which the proPO system is activated upon pathogen invasion. Human and insect antimicrobial peptides share distinct structural and functional similarities, insects produce the majority of their AMPs from the fat body while mammals rely on production locally at the site of infection by epithelial/mucosal cells. Understanding the structure and function of the insect immune system and the similarities with the innate immune response of mammals will increase the attractiveness of using insects as in vivo models for studying host - pathogen interactions.
Keywords: Antimicrobial peptide; Toll; coagulation; humoral immunity; insect; mammal; melanization.
Figures



References
-
- Bergin D, Reeves EP, Renwick J, et al. Superoxide production in galleria mellonella hemocytes : identification of proteins homologous to the NADPH oxidase complex of human neutrophils superoxide production in galleria mellonella hemocytes : identification of proteins homologous to the NADPH Ox. Infect Immun. 2005;73:4161–4170. - PMC - PubMed
-
- Renwick J, Reeves EP, Wientjes FB, et al. Translocation of proteins homologous to human neutrophil p47phox and p67phox to the cell membrane in activated hemocytes of Galleria mellonella. Dev Comp Immunol. 2007;31:347–359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
