The effect of acute respiratory distress syndrome on bone marrow-derived mesenchymal stem cells
- PMID: 30257702
- PMCID: PMC6158906
- DOI: 10.1186/s13287-018-0981-3
The effect of acute respiratory distress syndrome on bone marrow-derived mesenchymal stem cells
Abstract
Background: It is known that, following a physiological insult, bone marrow-derived mesenchymal stem cells (MSCs) mobilize and home to the site of injury. However, the effect of injury on the function of endogenous MSCs is unknown. In this study, MSCs harvested from the bone marrow of swine with or without acute respiratory distress syndrome (ARDS) were assessed for their characteristics and therapeutic function.
Methods: MSCs were harvested from three groups of anesthetized and mechanically ventilated swine (n = 3 in each group): 1) no ARDS ('Uninjured' group); 2) ARDS induced via smoke inhalation and 40% burn and treated with inhaled epinephrine ('Injured Treated' group); and 3) ARDS without treatment ('Injured Untreated' group). Cellular evaluation of the three groups included: flow cytometry for MSC markers; colony forming unit-fibroblast (CFU-F) assay; proliferative and metabolic capacity; gene expression using quantitative real-time polymerase chain reaction (qRT-PCR); and a lipopolysaccharide (LPS) challenge, with or without coculture with mononuclear cells (MNCs), for evaluation of their protein secretion profile using Multiplex. Statistical analysis was performed using one- or two-way analysis of variance (ANOVA) with a Tukey's post-test; a p-value less than 0.05 was considered statistically significant.
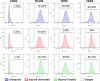
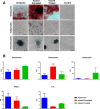
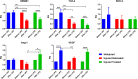
Results: Cells from all groups exhibited nearly 100% expression of MSC surface markers and retained their multidifferentiation capacity. However, the MSCs from the 'Injured Untreated' group generated a significantly higher number of colonies compared with the other two groups (p < 0.0001), indicative of increased clonogenic capacity following ARDS. Following an LPS challenge, the MSCs from the 'Injured Untreated' group exhibited a significant reduction in their proliferative capacity (p = 0.0002), significant downregulation in the expression of high-mobility group box 1 (HMGB1; p < 0.001), Toll-like receptor (TLR)-4 (p < 0.01), and vascular endothelial growth factor (VEGF; p < 0.05) genes, and significantly diminished secretory capacity for the inflammatory mediators interleukin (IL)-6 (p < 0.0001), IL-8 (p < 0.05), and tumor necrosis factor (TNF)-α (p < 0.05) compared with the 'Uninjured' group.
Conclusions: The results suggest that, following ARDS, there is an increase in the clonogenic capacity of MSCs to increase the available stem cell pool in vivo. However, MSCs harvested from subjects with ARDS seem to exhibit a diminished capacity to proliferate, express regenerative signals, and secrete pro/anti-inflammatory mediators.
Keywords: Acute respiratory distress syndrome (ARDS); Autologous therapy; Bone marrow; Immunomodulation; Injury; Mesenchymal stem cells (MSCs).
Conflict of interest statement
Ethics approval and consent to participate
Research was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council. The facility’s Institutional Animal Care and Use Committee approved all research conducted in this study. The facility where this research was conducted is fully accredited by AAALAC International.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

