Role of feasibility and pilot studies in randomised controlled trials: a cross-sectional study
- PMID: 30257847
- PMCID: PMC6169762
- DOI: 10.1136/bmjopen-2018-022233
Role of feasibility and pilot studies in randomised controlled trials: a cross-sectional study
Abstract
Objectives: To assess the value of pilot and feasibility studies to randomised controlled trials (RCTs) funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme. To explore the methodological components of pilot/feasibility studies and how they inform full RCTs.
Study design: Cross-sectional study.
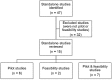
Setting: Both groups included NIHR HTA programme funded studies in the period 1 January 2010-31 December 2014 (decision date). Group 1: stand-alone pilot/feasibility studies published in the HTA Journal or accepted for publication. Group 2: all funded RCT applications funded by the HTA programme, including reference to an internal and/or external pilot/feasibility study. The methodological components were assessed using an adapted framework from a previous study.
Main outcome measures: The proportion of stand-alone pilot and feasibility studies which recommended proceeding to full trial and what study elements were assessed. The proportion of 'HTA funded' trials which used internal and external pilot and feasibility studies to inform the design of the trial.
Results: Group 1 identified 15 stand-alone pilot/feasibility studies. Study elements most commonly assessed were testing recruitment (100% in both groups), feasibility (83%, 100%) and suggestions for further study/investigation (83%, 100%). Group 2 identified 161 'HTA funded' applications: 59 cited an external pilot/feasibility study where testing recruitment (50%, 73%) and feasibility (42%, 73%) were the most commonly reported study elements: 92 reported an internal pilot/feasibility study where testing recruitment (93%, 100%) and feasibility (44%, 92%) were the most common study elements reported.
Conclusions: 'HTA funded' research which includes pilot and feasibility studies assesses a variety of study elements. Pilot and feasibility studies serve an important role when determining the most appropriate trial design. However, how they are reported and in what context requires caution when interpreting the findings and delivering a definitive trial.
Keywords: feasibility studies; health technology assessment; pilot studies; randomised controlled trials.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: ABJ and MAK are employed by the University of Southampton to work for NETSCC. ABJ is employed as the Senior Research Fellow for the Research on Research programme and has worked for NETSCC (and its predecessor organisation) in various roles since 2008. MAK is the scientific director at NETSCC and was an editor of the Health Technology Assessment journal. EK is employed by the University of Southampton and works at Southampton’s Clinical Trials Unit. EK worked for the Research on Research programme during June 2014 to December 2015. WP was a 4th Year BM5 Medicine student at the University of Southampton.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical