Structural basis, stoichiometry, and thermodynamics of binding of the chemokines KC and MIP2 to the glycosaminoglycan heparin
- PMID: 30257866
- PMCID: PMC6240865
- DOI: 10.1074/jbc.RA118.004866
Structural basis, stoichiometry, and thermodynamics of binding of the chemokines KC and MIP2 to the glycosaminoglycan heparin
Abstract
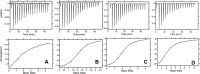
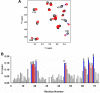
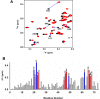
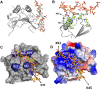
Keratinocyte-derived chemokine (KC or mCXCL1) and macrophage inflammatory protein 2 (MIP2 or mCXCL2) play nonredundant roles in trafficking blood neutrophils to sites of infection and injury. The functional responses of KC and MIP2 are intimately coupled to their interactions with glycosaminoglycans (GAGs). GAG interactions orchestrate chemokine concentration gradients and modulate receptor activity, which together regulate neutrophil trafficking. Here, using NMR, molecular dynamics (MD) simulations, and isothermal titration calorimetry (ITC), we characterized the molecular basis of KC and MIP2 binding to the GAG heparin. Both chemokines reversibly exist as monomers and dimers, and the NMR analysis indicates that the dimer binds heparin with higher affinity. The ITC experiments indicate a stoichiometry of two GAGs per KC or MIP2 dimer and that the enthalpic and entropic contributions vary significantly between the two chemokine-heparin complexes. NMR-based structural models of heparin-KC and heparin-MIP2 complexes reveal that different combinations of residues from the N-loop, 40s turn, β3-strand, and C-terminal helix form a binding surface within a monomer and that both conserved residues and residues unique to a particular chemokine mediate the binding interactions. MD simulations indicate significant residue-specific differences in their contribution to binding and affinity for a given chemokine and between chemokines. On the basis of our observations that KC and MIP2 bind to GAG via distinct molecular interactions, we propose that the differences in these GAG interactions lead to differences in neutrophil recruitment and play nonoverlapping roles in resolution of inflammation.
Keywords: chemokine; glycosaminoglycan; heparin; isothermal titration calorimetry (ITC); molecular dynamics; nuclear magnetic resonance (NMR).
© 2018 Sepuru et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Neutrophil recruitment by chemokines Cxcl1/KC and Cxcl2/MIP2: Role of Cxcr2 activation and glycosaminoglycan interactions.J Leukoc Biol. 2021 Apr;109(4):777-791. doi: 10.1002/JLB.3A0820-207R. Epub 2020 Sep 2. J Leukoc Biol. 2021. PMID: 32881070 Free PMC article.
-
Structural basis of a chemokine heterodimer binding to glycosaminoglycans.Biochem J. 2021 Mar 12;478(5):1009-1021. doi: 10.1042/BCJ20200927. Biochem J. 2021. PMID: 33463672
-
Molecular Basis of Chemokine CXCL5-Glycosaminoglycan Interactions.J Biol Chem. 2016 Sep 23;291(39):20539-50. doi: 10.1074/jbc.M116.745265. Epub 2016 Jul 28. J Biol Chem. 2016. PMID: 27471273 Free PMC article.
-
Glycosaminoglycan Interactions Fine-Tune Chemokine-Mediated Neutrophil Trafficking: Structural Insights and Molecular Mechanisms.J Histochem Cytochem. 2018 Apr;66(4):229-239. doi: 10.1369/0022155417739864. Epub 2018 Jan 1. J Histochem Cytochem. 2018. PMID: 29290145 Free PMC article. Review.
-
Targeting Chemokine-Glycosaminoglycan Interactions to Inhibit Inflammation.Front Immunol. 2020 Mar 31;11:483. doi: 10.3389/fimmu.2020.00483. eCollection 2020. Front Immunol. 2020. PMID: 32296423 Free PMC article. Review.
Cited by
-
Neutrophil recruitment by chemokines Cxcl1/KC and Cxcl2/MIP2: Role of Cxcr2 activation and glycosaminoglycan interactions.J Leukoc Biol. 2021 Apr;109(4):777-791. doi: 10.1002/JLB.3A0820-207R. Epub 2020 Sep 2. J Leukoc Biol. 2021. PMID: 32881070 Free PMC article.
-
Combinatorial Virtual Library Screening Study of Transforming Growth Factor-β2-Chondroitin Sulfate System.Int J Mol Sci. 2021 Jul 14;22(14):7542. doi: 10.3390/ijms22147542. Int J Mol Sci. 2021. PMID: 34299163 Free PMC article.
-
Studying specificity in protein-glycosaminoglycan recognition with umbrella sampling.Beilstein J Org Chem. 2023 Dec 19;19:1933-1946. doi: 10.3762/bjoc.19.144. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 38170083 Free PMC article.
-
Glycosaminoglycan-Protein Interactions and Their Roles in Human Disease.Front Mol Biosci. 2021 Mar 9;8:639666. doi: 10.3389/fmolb.2021.639666. eCollection 2021. Front Mol Biosci. 2021. PMID: 33768117 Free PMC article. Review.
-
Structural Insights Into How Proteoglycans Determine Chemokine-CXCR1/CXCR2 Interactions: Progress and Challenges.Front Immunol. 2020 Apr 24;11:660. doi: 10.3389/fimmu.2020.00660. eCollection 2020. Front Immunol. 2020. PMID: 32391006 Free PMC article. Review.
References
-
- Bonecchi R., Galliera E., Borroni E. M., Corsi M. M., Locati M., and Mantovani A. (2009) Chemokines and chemokine receptors: an overview. Front. Biosci. (Landmark Ed.) 14, 540–551 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

