Evolution of the calcium feedback steps of vertebrate phototransduction
- PMID: 30257895
- PMCID: PMC6170504
- DOI: 10.1098/rsob.180119
Evolution of the calcium feedback steps of vertebrate phototransduction
Abstract
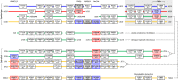
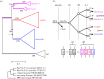
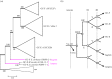
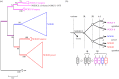
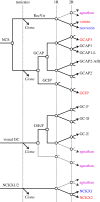
We examined the genes encoding the proteins that mediate the Ca-feedback regulatory system in vertebrate rod and cone phototransduction. These proteins comprise four families: recoverin/visinin, the guanylyl cyclase activating proteins (GCAPs), the guanylyl cyclases (GCs) and the sodium/calcium-potassium exchangers (NCKXs). We identified a paralogon containing at least 36 phototransduction genes from at least fourteen families, including all four of the families involved in the Ca-feedback loop (recoverin/visinin, GCAPs, GCs and NCKXs). By combining analyses of gene synteny with analyses of the molecular phylogeny for each of these four families of genes for Ca-feedback regulation, we have established the likely pattern of gene duplications and losses underlying the expansion of isoforms, both before and during the two rounds of whole-genome duplication (2R WGD) that occurred in early vertebrate evolution. Furthermore, by combining our results with earlier evidence on the timing of duplication of the visual G-protein receptor kinase genes, we propose that specialization of proto-vertebrate photoreceptor cells for operation at high and low light intensities preceded the emergence of rhodopsin, which occurred during 2R WGD.
Keywords: evolution; guanylyl cyclase; guanylyl cyclase activating protein; phototransduction; recoverin; sodium/calcium–potassium exchanger.
© 2018 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures



Similar articles
-
Selective Gene Loss of Visual and Olfactory Guanylyl Cyclase Genes Following the Two Rounds of Vertebrate-Specific Whole-Genome Duplications.Genome Biol Evol. 2020 Nov 3;12(11):2153-2167. doi: 10.1093/gbe/evaa192. Genome Biol Evol. 2020. PMID: 32915957 Free PMC article.
-
The vertebrate ancestral repertoire of visual opsins, transducin alpha subunits and oxytocin/vasopressin receptors was established by duplication of their shared genomic region in the two rounds of early vertebrate genome duplications.BMC Evol Biol. 2013 Nov 2;13:238. doi: 10.1186/1471-2148-13-238. BMC Evol Biol. 2013. PMID: 24180662 Free PMC article.
-
Evolution of the genes mediating phototransduction in rod and cone photoreceptors.Prog Retin Eye Res. 2020 May;76:100823. doi: 10.1016/j.preteyeres.2019.100823. Epub 2019 Nov 29. Prog Retin Eye Res. 2020. PMID: 31790748 Review.
-
Guanylate cyclase-activating protein 2 contributes to phototransduction and light adaptation in mouse cone photoreceptors.J Biol Chem. 2018 May 11;293(19):7457-7465. doi: 10.1074/jbc.RA117.001574. Epub 2018 Mar 16. J Biol Chem. 2018. PMID: 29549122 Free PMC article.
-
Regulation of photoreceptor membrane guanylyl cyclases by guanylyl cyclase activator proteins.Methods. 1999 Dec;19(4):521-31. doi: 10.1006/meth.1999.0894. Methods. 1999. PMID: 10581151 Review.
Cited by
-
Inhibition of high level E2F in a RB1 proficient MYCN overexpressing chicken retinoblastoma model normalizes neoplastic behaviour.Cell Oncol (Dordr). 2024 Feb;47(1):209-227. doi: 10.1007/s13402-023-00863-0. Epub 2023 Aug 22. Cell Oncol (Dordr). 2024. PMID: 37606819 Free PMC article.
-
Regulation of retinal membrane guanylyl cyclase (RetGC) by negative calcium feedback and RD3 protein.Pflugers Arch. 2021 Sep;473(9):1393-1410. doi: 10.1007/s00424-021-02523-4. Epub 2021 Feb 3. Pflugers Arch. 2021. PMID: 33537894 Free PMC article. Review.
-
Selective Gene Loss of Visual and Olfactory Guanylyl Cyclase Genes Following the Two Rounds of Vertebrate-Specific Whole-Genome Duplications.Genome Biol Evol. 2020 Nov 3;12(11):2153-2167. doi: 10.1093/gbe/evaa192. Genome Biol Evol. 2020. PMID: 32915957 Free PMC article.
-
A comparative analysis of gene and protein expression in chronic and acute models of photoreceptor degeneration in adult zebrafish.Front Cell Dev Biol. 2023 Sep 7;11:1233269. doi: 10.3389/fcell.2023.1233269. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37745292 Free PMC article.
-
Evolution of visual guanylyl cyclases and their activating proteins with respect to clade and species-specific visual system adaptation.Front Mol Neurosci. 2023 Mar 16;16:1131093. doi: 10.3389/fnmol.2023.1131093. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37008786 Free PMC article.
References
-
- Ohno S. 1970. Evolution by gene duplication. London, UK: Allen and Unwin.
-
- Lagman D, Ocampo Daza D, Widmark J, Abalo XM, Sundström G, Larhammar D. 2013. The vertebrate ancestral repertoire of visual opsins, transducin alpha subunits and oxytocin/vasopressin receptors was established by duplication of their shared genomic region in the two rounds of early vertebrate genome duplications. BMC Evol. Biol. 13, 238 (10.1186/1471-2148-13-238) - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

