An innovative ovipositor for niche exploitation impacts genital coevolution between sexes in a fruit-damaging Drosophila
- PMID: 30257912
- PMCID: PMC6170814
- DOI: 10.1098/rspb.2018.1635
An innovative ovipositor for niche exploitation impacts genital coevolution between sexes in a fruit-damaging Drosophila
Abstract
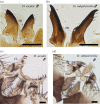
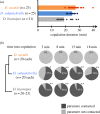
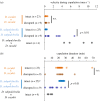
Limited attention has been given to ecological factors influencing the coevolution of male and female genitalia. The innovative ovipositor of Drosophila suzukii, an invading fruit pest, represents an appealing case to document this phenomenon. The serrated saw-like ovipositor is used to pierce the hard skin of ripening fruits that are not used by other fruit flies that prefer soft decaying fruits. Here, we highlight another function of the ovipositor related to its involvement in genital coupling during copulation. We compared the morphology and coupling of male and female genitalia in this species to its sibling species, Drosophila subpulchrella, and to an outgroup species, Drosophila biarmipes These comparisons and a surgical manipulation indicated that the shape of male genitalia in D. suzukii has had to be adjusted to ensure tight coupling, despite having to abandon the use of a hook-like structure, paramere, because of the more linearly elongated ovipositor. This phenomenon demonstrates that ecological niche exploitation can directly affect the mechanics of genital coupling and potentially cause incompatibility among divergent forms. This model case provides new insights towards elucidating the importance of the dual functions of ovipositors in other insect species that potentially induce genital coevolution and ecological speciation.
Keywords: Drosophila subpulchrella; Drosophila suzukii; coevolution between the sexes; mechanical incompatibility; morphology of genitalia.
© 2018 The Author(s).
Conflict of interest statement
We have no competing interests.
Figures


References
-
- Eberhard WG. 1985. Sexual selection and animal genitalia. Cambridge, MA: Harvard University Press.
-
- Simmons LW. 2014. Sexual selection and genital evolution. Aust. Entomol. 53, 1–17. ( 10.1111/aen.12053) - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
