Protein Isoprenylation in Yeast Targets COOH-Terminal Sequences Not Adhering to the CaaX Consensus
- PMID: 30257935
- PMCID: PMC6283164
- DOI: 10.1534/genetics.118.301454
Protein Isoprenylation in Yeast Targets COOH-Terminal Sequences Not Adhering to the CaaX Consensus
Abstract
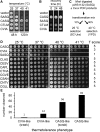
Protein isoprenylation targets a subset of COOH-terminal Cxxx tetrapeptide sequences that has been operationally defined as a CaaX motif. The specificity of the farnesyl transferase toward each of the possible 8000 combinations of Cxxx sequences, however, remains largely unresolved. In part, it has been difficult to consolidate results stemming from in vitro and in silico approaches that yield a wider array of prenylatable sequences relative to those known in vivo We have investigated whether this disconnect results from the multistep complexity of post-translational modification that occurs in vivo to CaaX proteins. For example, the Ras GTPases undergo isoprenylation followed by additional proteolysis and carboxymethylation events at the COOH-terminus. By contrast, Saccharomyces cerevisiae Hsp40 Ydj1p is isoprenylated but not subject to additional modification. In fact, additional modifications are detrimental to Ydj1p activity in vivo We have taken advantage of the properties of Ydj1p and a Ydj1p-dependent growth assay to identify sequences that permit Ydj1p isoprenylation in vivo while simultaneously selecting against nonprenylatable and more extensively modified sequences. The recovered sequences are largely nonoverlapping with those previously identified using an in vivo Ras-based yeast reporter. Moreover, most of the sequences are not readily predicted as isoprenylation targets by existing prediction algorithms. Our results reveal that the yeast CaaX-type prenyltransferases can utilize a range of sequence combinations that extend beyond the traditional constraints for CaaX proteins, which implies that more proteins may be isoprenylated than previously considered.
Keywords: CaaX; Hsp40; farnesyl transferase; isoprenylation; thermotolerance.
Copyright © 2018 by the Genetics Society of America.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

