Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome
- PMID: 30257952
- PMCID: PMC6166472
- DOI: 10.1126/scitranslmed.aat7163
Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome
Abstract
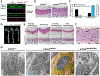
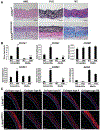
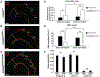
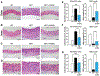
Hutchinson-Gilford progeria syndrome is a disorder of premature aging in children caused by de novo mutations in LMNA that lead to the synthesis of an internally truncated form of prelamin A (commonly called progerin). The production of progerin causes multiple disease phenotypes, including an unusual vascular phenotype characterized by the loss of smooth muscle cells in the arterial media and fibrosis of the adventitia. We show that progerin expression, combined with mechanical stress, promotes smooth muscle cell death. Disrupting the linker of the nucleoskeleton and cytoskeleton (LINC) complex in smooth muscle cells ameliorates the toxic effects of progerin on smooth muscle cells and limits the accompanying adventitial fibrosis.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


References
-
- DeBusk FL, The Hutchinson-Gilford progeria syndrome. Report of 4 cases and review of the literature. J. Pediatrics 80, 697–724 (1972). - PubMed
-
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS, Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298 (2003). - PMC - PubMed
-
- Kieran MW, Gordon L, Kleinman M, New approaches to progeria. Pediatrics 120, 834–841 (2007). - PubMed
-
- Gordon LB, McCarten KM, Giobbie-Hurder A, Machan JT, Campbell SE, Berns SD, Kieran MW, Disease progression in Hutchinson-Gilford progeria syndrome: impact on growth and development. Pediatrics 120, 824–833 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

