Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections
- PMID: 30257953
- PMCID: PMC6637966
- DOI: 10.1126/scitranslmed.aat7520
Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections
Abstract
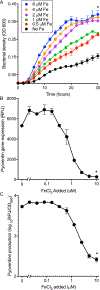
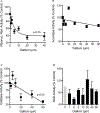
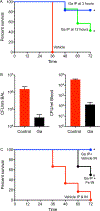
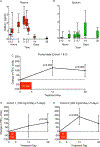
The lack of new antibiotics is among the most critical challenges facing medicine. The problem is particularly acute for Gram-negative bacteria. An unconventional antibiotic strategy is to target bacterial nutrition and metabolism. The metal gallium can disrupt bacterial iron metabolism because it substitutes for iron when taken up by bacteria. We investigated the antibiotic activity of gallium ex vivo, in a mouse model of airway infection, and in a phase 1 clinical trial in individuals with cystic fibrosis (CF) and chronic Pseudomonas aeruginosa airway infections. Our results show that micromolar concentrations of gallium inhibited P. aeruginosa growth in sputum samples from patients with CF. Ex vivo experiments indicated that gallium inhibited key iron-dependent bacterial enzymes and increased bacterial sensitivity to oxidants. Furthermore, gallium resistance developed slowly, its activity was synergistic with certain antibiotics, and gallium did not diminish the antibacterial activity of host macrophages. Systemic gallium treatment showed antibiotic activity in murine lung infections. In addition, systemic gallium treatment improved lung function in people with CF and chronic P. aeruginosa lung infection in a preliminary phase 1 clinical trial. These findings raise the possibility that human infections could be treated by targeting iron metabolism or other nutritional vulnerabilities of bacterial pathogens.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
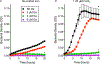



References
-
- Flamm RK, Weaver MK, Thornsberry C, Jones ME, Karlowsky JA, Sahm DF, Factors associated with relative rates of antibiotic resistance in Pseudomonas aeruginosa isolates tested in clinical laboratories in the United States from 1999 to 2002. Antimicrob Agents Chemother 48, 2431–2436 (2004); published online EpubJul ( - PMC - PubMed
-
- Skovgaard N, New trends in emerging pathogens. Int J Food Microbiol 120, 217–224 (2007); published online EpubDec 15 ( - PubMed
-
- Stewart PS, Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292, 107–113 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

