PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production
- PMID: 30257954
- PMCID: PMC6263177
- DOI: 10.1126/scitranslmed.aar8356
PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production
Abstract
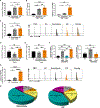
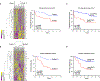
Pulmonary fibrosis is a progressive inflammatory disease with high mortality and limited therapeutic options. Previous genetic and immunologic investigations suggest common intersections between idiopathic pulmonary fibrosis (IPF), sarcoidosis, and murine models of pulmonary fibrosis. To identify immune responses that precede collagen deposition, we conducted molecular, immunohistochemical, and flow cytometric analysis of human and murine specimens. Immunohistochemistry revealed programmed cell death-1 (PD-1) up-regulation on IPF lymphocytes. PD-1+CD4+ T cells with reduced proliferative capacity and increased transforming growth factor-β (TGF-β)/interleukin-17A (IL-17A) expression were detected in IPF, sarcoidosis, and bleomycin CD4+ T cells. PD-1+ T helper 17 cells are the predominant CD4+ T cell subset expressing TGF-β. Coculture of PD-1+CD4+ T cells with human lung fibroblasts induced collagen-1 production. Strikingly, ex vivo PD-1 pathway blockade resulted in reductions in TGF-β and IL-17A expression from CD4+ T cells, with concomitant declines in collagen-1 production from fibroblasts. Molecular analysis demonstrated PD-1 regulation of the transcription factor STAT3 (signal transducer and activator of transcription 3). Chemical blockade of STAT3, using the inhibitor STATTIC, inhibited collagen-1 production. Both bleomycin administration to PD-1 null mice or use of antibody against programmed cell death ligand 1 (PD-L1) demonstrated significantly reduced fibrosis compared to controls. This work identifies a critical, previously unrecognized role for PD-1+CD4+ T cells in pulmonary fibrosis, supporting the use of readily available therapeutics that directly address interstitial lung disease pathophysiology.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures





References
-
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD, The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med 14, 45–54 (2008). - PubMed
-
- King TE Jr., Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW, A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med 370, 2083–2092 (2014). - PubMed
-
- Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, Wilke CA, Chrisman CJ, Moore BB, Protection from fluorescein isothiocyanateinduced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J. Immunol 172, 4068–4076 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL092870/HL/NHLBI NIH HHS/United States
- K08 HL130595/HL/NHLBI NIH HHS/United States
- R01 HL122554/HL/NHLBI NIH HHS/United States
- R01 HL113326/HL/NHLBI NIH HHS/United States
- R21 AI121420/AI/NIAID NIH HHS/United States
- R01 AI111820/AI/NIAID NIH HHS/United States
- R01 DK104817/DK/NIDDK NIH HHS/United States
- U19 AI095227/AI/NIAID NIH HHS/United States
- R01 HL130796/HL/NHLBI NIH HHS/United States
- P30 GM110766/GM/NIGMS NIH HHS/United States
- R01 HL117074/HL/NHLBI NIH HHS/United States
- K24 HL127301/HL/NHLBI NIH HHS/United States
- R01 AI124456/AI/NIAID NIH HHS/United States
- T32 HL094296/HL/NHLBI NIH HHS/United States
- I01 BX000624/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

