Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant
- PMID: 30257956
- PMCID: PMC6468978
- DOI: 10.1126/scitranslmed.aap9489
Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant
Abstract
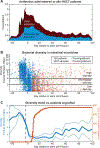
Antibiotic treatment can deplete the commensal bacteria of a patient's gut microbiota and, paradoxically, increase their risk of subsequent infections. In allogeneic hematopoietic stem cell transplantation (allo-HSCT), antibiotic administration is essential for optimal clinical outcomes but significantly disrupts intestinal microbiota diversity, leading to loss of many beneficial microbes. Although gut microbiota diversity loss during allo-HSCT is associated with increased mortality, approaches to reestablish depleted commensal bacteria have yet to be developed. We have initiated a randomized, controlled clinical trial of autologous fecal microbiota transplantation (auto-FMT) versus no intervention and have analyzed the intestinal microbiota profiles of 25 allo-HSCT patients (14 who received auto-FMT treatment and 11 control patients who did not). Changes in gut microbiota diversity and composition revealed that the auto-FMT intervention boosted microbial diversity and reestablished the intestinal microbiota composition that the patient had before antibiotic treatment and allo-HSCT. These results demonstrate the potential for fecal sample banking and posttreatment remediation of a patient's gut microbiota after microbiota-depleting antibiotic treatment during allo-HSCT.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



References
-
- Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A, Perales M-A, Jenq RR, van den Brink MRM, Pamer EG, Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis 55, 905–914 (2012). - PMC - PubMed
-
- Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, Zhu W, Sporrer D, Hehlgans T, Kreutz M, Holler B, Wolff D, Edinger M, Andreesen R, Levine JE, Ferrara JL, Gessner A, Spang R, Oefner PJ, Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant 20, 640–645 (2014). - PMC - PubMed
-
- Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, Littmann ER, Ling L, Gobourne AC, Miller LC, Docampo MD, Peled JU, Arpaia N, Cross JR, Peets TK, Lumish MA, Shono Y, Dudakov JA, Poeck H, Hanash AM, Barker JN, Perales M-A, Giralt SA, Pamer EG, van den Brink MRM, Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transplant 21, 1373–1383 (2015). - PMC - PubMed
-
- Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, Gobourne A, Lipuma L, Young LF, Smith OM, Ghosh A, Hanash AM, Goldberg JD, Aoyama K, Blazar BR, Pamer EG, van den Brink MRM, Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med 209, 903–911 (2012). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

