Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling
- PMID: 30258047
- PMCID: PMC6158290
- DOI: 10.1038/s41419-018-1041-8
Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling
Abstract
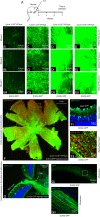
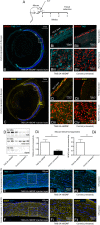
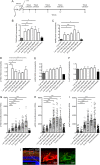
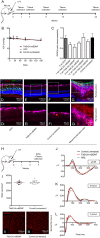
Previous studies have demonstrated that intravitreal delivery of brain-derived neurotrophic factor (BDNF) by injection of recombinant protein or by gene therapy can alleviate retinal ganglion cell (RGC) loss after optic nerve injury. BDNF gene therapy can improve RGC survival in experimental models of glaucoma, the leading cause of irreversible blindness worldwide. However, the therapeutic efficacy of BDNF supplementation alone is time limited at least in part due to BDNF receptor downregulation. Tropomyosin-related receptor kinase-B (TrkB) downregulation has been reported in many neurological diseases including glaucoma, potentially limiting the effect of sustained or repeated BDNF delivery.Here, we characterize a novel adeno-associated virus (AAV) gene therapy (AAV2 TrkB-2A-mBDNF) that not only increases BDNF production but also improves long-term neuroprotective signaling by increasing expression of the BDNF receptor (TrkB) within the inner retina. This approach leads to significant and sustained elevation of survival signaling pathways ERK and AKT within RGCs over 6 months and avoids the receptor downregulation which we observe with treatment with AAV2 BDNF alone. We validate the neuroprotective efficacy of AAV2 TrkB-2A-mBDNF in a mouse model of optic nerve injury, where it outperforms conventional AAV2 BDNF or AAV2 TrkB therapy, before showing powerful proof of concept neuroprotection of RGCs and axons in a rat model of chronic intraocular pressure (IOP) elevation. We also show that there are no adverse effects of the vector on retinal structure or function as assessed by histology and electroretinography in young or aged animals. Further studies are underway to explore the potential of this vector as a candidate for progression into clinical studies to protect RGCs in patients with glaucoma and progressive visual loss despite conventional IOP-lowering treatment.
Conflict of interest statement
P.S.W. and K.R.M. have a financial interest in Quethera Ltd, a company working to develop gene therapy approaches to neurodegenerative diseases. A.O., K.H., and K.R.M. have received grant support from Quethera Ltd. T.Z.K., L.S., A.C.B., and G.Y.X.K. have no disclosures.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

