Asymmetric remote C-H borylation of internal alkenes via alkene isomerization
- PMID: 30258070
- PMCID: PMC6158179
- DOI: 10.1038/s41467-018-06240-y
Asymmetric remote C-H borylation of internal alkenes via alkene isomerization
Abstract
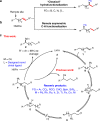
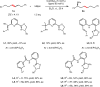
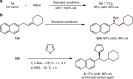
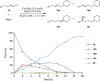
Recent years have witnessed the growing interest in the remote functionalization of alkenes for it offers a strategy to activate the challenging C-H bonds distant from the initiation point via alkene isomerization/functionalization. However, the catalytic enantioselective isomerization/functionalization with one single transition metal catalyst remains rare. Here we report a highly regio- and enantioselective cobalt-catalyzed remote C-H bond borylation of internal alkenes via sequential alkene isomerization/hydroboration. A chiral ligand featured twisted pincer, anionic, and non-rigid characters is designed and used for this transformation. This methodology, which is operationally simple using low catalyst loading without additional activator, shows excellent enantioselectivity and can be used to convert various internal alkenes with regio- and stereoisomers to valuable chiral secondary organoboronates with good functional group tolerance.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

