Heterocyclic sterol probes for live monitoring of sterol trafficking and lysosomal storage disorders
- PMID: 30258093
- PMCID: PMC6158244
- DOI: 10.1038/s41598-018-32776-6
Heterocyclic sterol probes for live monitoring of sterol trafficking and lysosomal storage disorders
Abstract
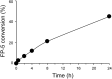
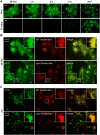
The monitoring of intracellular cholesterol homeostasis and trafficking is of great importance because their imbalance leads to many pathologies. Reliable tools for cholesterol detection are in demand. This study presents the design and synthesis of fluorescent probes for cholesterol recognition and demonstrates their selectivity by a variety of methods. The construction of dedicated library of 14 probes was based on heterocyclic (pyridine)-sterol derivatives with various attached fluorophores. The most promising probe, a P1-BODIPY conjugate FP-5, was analysed in detail and showed an intensive labelling of cellular membranes followed by intracellular redistribution into various cholesterol rich organelles and vesicles. FP-5 displayed a stronger signal, with faster kinetics, than the commercial TF-Chol probe. In addition, cells with pharmacologically disrupted cholesterol transport, or with a genetic mutation of cholesterol transporting protein NPC1, exhibited strong and fast FP-5 signal in the endo/lysosomal compartment, co-localizing with filipin staining of cholesterol. Hence, FP-5 has high potential as a new probe for monitoring cholesterol trafficking and its disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Influence of fluorophore and linker length on the localization and trafficking of fluorescent sterol probes.Sci Rep. 2020 Dec 16;10(1):22053. doi: 10.1038/s41598-020-78085-9. Sci Rep. 2020. PMID: 33328481 Free PMC article.
-
Use of BODIPY-Cholesterol (TF-Chol) for Visualizing Lysosomal Cholesterol Accumulation.Traffic. 2016 Sep;17(9):1054-7. doi: 10.1111/tra.12414. Epub 2016 Jun 13. Traffic. 2016. PMID: 27187581 Free PMC article.
-
Membrane orientation and lateral diffusion of BODIPY-cholesterol as a function of probe structure.Biophys J. 2013 Nov 5;105(9):2082-92. doi: 10.1016/j.bpj.2013.09.031. Biophys J. 2013. PMID: 24209853 Free PMC article.
-
Following intracellular cholesterol transport by linear and non-linear optical microscopy of intrinsically fluorescent sterols.Curr Pharm Biotechnol. 2012 Feb;13(2):303-18. doi: 10.2174/138920112799095301. Curr Pharm Biotechnol. 2012. PMID: 21470123 Review.
-
Potential of BODIPY-cholesterol for analysis of cholesterol transport and diffusion in living cells.Chem Phys Lipids. 2016 Jan;194:12-28. doi: 10.1016/j.chemphyslip.2015.08.007. Epub 2015 Aug 17. Chem Phys Lipids. 2016. PMID: 26291493 Review.
Cited by
-
Sterolight as imaging tool to study sterol uptake, trafficking and efflux in living cells.Sci Rep. 2022 Apr 15;12(1):6264. doi: 10.1038/s41598-022-10134-x. Sci Rep. 2022. PMID: 35428843 Free PMC article.
-
Cyclodextrins inhibit TRPV1 and TRPA1 activation-induced nociception via cholesterol depletion.J Lipid Res. 2025 Jul;66(7):100844. doi: 10.1016/j.jlr.2025.100844. Epub 2025 Jun 16. J Lipid Res. 2025. PMID: 40532876 Free PMC article.
-
Cyclodextrin derivatives decrease Transient Receptor Potential vanilloid 1 and Ankyrin 1 ion channel activation via altering the surrounding membrane microenvironment by cholesterol depletion.Front Cell Dev Biol. 2024 Feb 28;12:1334130. doi: 10.3389/fcell.2024.1334130. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38481530 Free PMC article.
-
Influence of fluorophore and linker length on the localization and trafficking of fluorescent sterol probes.Sci Rep. 2020 Dec 16;10(1):22053. doi: 10.1038/s41598-020-78085-9. Sci Rep. 2020. PMID: 33328481 Free PMC article.
-
Lipid Metabolism and Immune Function: Chemical Tools for Insights into T-Cell Biology.Chembiochem. 2025 Jun 3;26(11):e202400980. doi: 10.1002/cbic.202400980. Epub 2025 Apr 16. Chembiochem. 2025. PMID: 40162512 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 17-02836S/Grantová Agentura České Republiky (Grant Agency of the Czech Republic)/International
- LO1220/Ministerstvo Školství, Mládeže a Tělovýchovy (Ministry of Education, Youth and Sports)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

