Perineuronal nets in the lateral hypothalamus area regulate cue-induced reinstatement of cocaine-seeking behavior
- PMID: 30258113
- PMCID: PMC6461795
- DOI: 10.1038/s41386-018-0212-8
Perineuronal nets in the lateral hypothalamus area regulate cue-induced reinstatement of cocaine-seeking behavior
Abstract
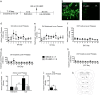
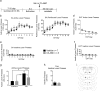
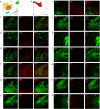
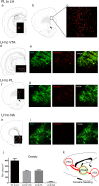
We previously reported that a small, circumscribed region of the lateral hypothalamus, the anterior dorsal region (LHAad), stains heavily for PNNs and dense extracellular matrix (PNNs/ECM) with Wisteria floribunda agglutinin (WFA), and critically contributes to the acquisition of cocaine-induced conditioned place preference and cocaine self-administration. Here we tested the role of LHAad PNNs/ECM in cue-induced reinstatement in cocaine self-administering (SA) rats and identified how it is embedded in the circuitry of motivated behavior and drug reward. Degradation of PNNs/ECM in the LHAad using chondroitinase ABC (Ch-ABC) blocked the expression of cue-induced reinstatement of cocaine- but not sucrose-seeking behavior. We also identified for the first time the phenotype of LHAad PNN/ECM-surrounded neurons. LHAad neurons co-localized mainly with parvalbumin (PV+) and GABA. Predominant co-localization of WFA with VGLUT2 and GABA but not with GAD65/67 or glutamate indicates that the PNN/ECM-rich LHAad is predominantly GABAergic and receives dense glutamatergic input. The LHAad did not express significant amounts of melanin-concentrating hormone (MCH), orexin, or galanin; neuropeptides that regulate both food-induced and cocaine-induced behavior. In addition, retrobead injections demonstrated that the LHAad receives robust prelimbic prefrontal cortex (PFC) input and provides moderate input to the prelimbic PFC and ventral tegmental area (VTA), with no apparent input to the nucleus accumbens. In summary, the dense PNN/ECM zone in the LHAad embedded within the circuitry associated with reward pinpoints a novel region that controls the expression of cocaine-seeking behavior.
Conflict of interest statement
The authors declare no competing interests.
Figures
Comment in
-
Break the net, break the cycle: removal of perineuronal nets in the lateral hypothalamus decreases cocaine relapse.Neuropsychopharmacology. 2019 Apr;44(5):835-836. doi: 10.1038/s41386-018-0245-z. Epub 2018 Oct 24. Neuropsychopharmacology. 2019. PMID: 30867569 Free PMC article. No abstract available.
References
-
- Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M, et al. Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology. 2011;35:2120–33. doi: 10.1038/npp.2010.90. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- K99 DA045082/DA/NIDA NIH HHS/United States
- R01 DA040965/DA/NIDA NIH HHS/United States
- DA033404/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
- R01 DA033404/DA/NIDA NIH HHS/United States
- DA040965/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

