Neprilysin inhibition promotes corneal wound healing
- PMID: 30258206
- PMCID: PMC6158251
- DOI: 10.1038/s41598-018-32773-9
Neprilysin inhibition promotes corneal wound healing
Abstract
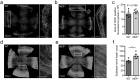
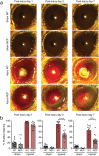
Neprilysin (NEP), an ectoenzyme that modulates inflammation by degrading neuropeptides, was recently identified in the human corneal epithelium. The cornea expresses many NEP substrates, but the function of NEP in homeostatic maintenance and wound healing of the cornea is unknown. We therefore investigated the role of this enzyme under naive and injured conditions using NEP-deficient (NEP-/-) and wild type (WT) control mice. In vivo ocular surface imaging and histological analysis of corneal tissue showed no differences in limbal vasculature or corneal anatomy between naive NEP-/- and WT mice. Histological examination revealed increased corneal innervation in NEP-/- mice. In an alkali burn model of corneal injury, corneal wound healing was significantly accelerated in NEP-/- mice compared to WT controls 3 days after injury. Daily intraperitoneal administration of the NEP inhibitor thiorphan also accelerated corneal wound healing after alkali injury in WT mice. Collectively, our data identify a previously unknown role of NEP in the cornea, in which pharmacologic inhibition of its activity may provide a novel therapeutic option for patients with corneal injury.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

