Lab-on-a-Chip: Frontier Science in the Classroom
- PMID: 30258250
- PMCID: PMC6150665
- DOI: 10.1021/acs.jchemed.7b00506
Lab-on-a-Chip: Frontier Science in the Classroom
Abstract
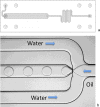
Lab-on-a-chip technology is brought into the classroom through development of a lesson series with hands-on practicals. Students can discover the principles of microfluidics with different practicals covering laminar flow, micromixing, and droplet generation, as well as trapping and counting beads. A quite affordable novel production technique using scissor-cut and laser-cut lamination sheets is presented, which provides good insight into how scientific lab-on-a-chip devices are produced. In this way high school students can now produce lab-on-a-chip devices using lamination sheets and their own lab-on-a-chip design. We begin with a review of previous reports on the use of lab-on-a-chip technology in classrooms, followed by an overview of the practicals and projects we have developed with student safety in mind. We conclude with an educational scenario and some initial promising results for student learning outcomes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Bridle H.; Morton J.; Cameron P.; Desmulliez M. P. Y.; Kersaudy- Kerhoas M. Design of problem-based learning activities in the field of microfluidics for 12- to 13-year-old participants—Small Plumbing!: empowering the next generation of microfluidic engineers. Microfluid. Nanofluid. 2016, 20, 103. 10.1007/s10404-016-1770-x. - DOI
-
- Piunno P. A. E.; Zetina A.; Chu N.; Tavares A. J.; Noor M. O.; Petryayeva E.; Uddayasankar U.; Veglio A. A comprehensive microfluidics device construction and characterization module for the advanced undergraduate analytical chemistry laboratory. J. Chem. Educ. 2014, 91, 902–907. 10.1021/ed400728a. - DOI
-
- Chia M. C.; Sweeney C. M.; Odom T. W. Chemistry in microfluidic channels. J. Chem. Educ. 2011, 88, 461–464. 10.1021/ed1008624. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
