Generating Human Organs via Interspecies Chimera Formation: Advances and Barriers
- PMID: 30258320
- PMCID: PMC6153627
Generating Human Organs via Interspecies Chimera Formation: Advances and Barriers
Abstract
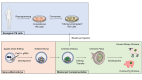
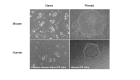
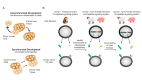
The shortage of human organs for transplantation is a devastating medical problem. One way to expand organ supply is to derive functional organs from patient-specific stem cells. Due to their capacity to grow indefinitely in the laboratory and differentiate into any cell type of the human body, patient-specific pluripotent stem (PS) cells harbor the potential to provide an inexhaustible supply of donor cells for transplantation. However, current efforts to generate functional organs from PS cells have so far been unsuccessful. An alternative and promising strategy is to generate human organs inside large animal species through a technique called interspecies blastocyst complementation. In this method, animals comprised of cells from human and animal species are generated by injecting donor human PS cells into animal host embryos. Critical genes for organ development are knocked out by genome editing, allowing donor human PS cells to populate the vacated niche. In principle, this experimental approach will produce a desired organ of human origin inside a host animal. In this mini-review, we focus on recent advances that may bring the promise of blastocyst complementation to clinical practice. While CRISPR/Cas9 has accelerated the creation of transgenic large animals such as pigs and sheep, we propose that further advances in the generation of chimera-competent human PS cells are needed to achieve interspecies blastocyst complementation. It will also be necessary to define the constituents of the species barrier, which inhibits efficient colonization of host animal embryos with human cells. Interspecies blastocyst complementation is a promising approach to help overcome the organ shortage facing the practice of clinical medicine today.
Keywords: CRISPR; Cas9; Pdx1; blastocyst complementation; chimeras; human pluripotent stem cells; interspecies blastocyst complementation; interspecies chimeras; mouse pluripotent stem cells; naive pluripotency; naive pluripotent stem cells; organ generation; organ shortage; organ transplantation; pluripotency; pluripotent stem cells; primed pluripotency; primed pluripotent stem cells; reprogramming.
Figures
Similar articles
-
ERK-independent African Green monkey pluripotent stem cells in a putative chimera-competent state.Biochem Biophys Res Commun. 2019 Feb 26;510(1):78-84. doi: 10.1016/j.bbrc.2019.01.037. Epub 2019 Jan 16. Biochem Biophys Res Commun. 2019. PMID: 30660369
-
Generation of human organs in pigs via interspecies blastocyst complementation.Reprod Domest Anim. 2016 Oct;51 Suppl 2:18-24. doi: 10.1111/rda.12796. Reprod Domest Anim. 2016. PMID: 27762052
-
Human-animal interspecies chimerism via blastocyst complementation: advances, challenges and perspectives: a narrative review.Stem Cell Investig. 2021 Oct 11;8:20. doi: 10.21037/sci-2020-074. eCollection 2021. Stem Cell Investig. 2021. PMID: 34815975 Free PMC article. Review.
-
The road to generating transplantable organs: from blastocyst complementation to interspecies chimeras.Development. 2021 Jun 15;148(12):dev195792. doi: 10.1242/dev.195792. Epub 2021 Jun 16. Development. 2021. PMID: 34132325 Free PMC article.
-
Parsing the pluripotency continuum in humans and non-human primates for interspecies chimera generation.Exp Cell Res. 2020 Feb 1;387(1):111747. doi: 10.1016/j.yexcr.2019.111747. Epub 2019 Nov 26. Exp Cell Res. 2020. PMID: 31778671 Review.
Cited by
-
Interspecies chimeric conditions affect the developmental rate of human pluripotent stem cells.PLoS Comput Biol. 2021 Mar 1;17(3):e1008778. doi: 10.1371/journal.pcbi.1008778. eCollection 2021 Mar. PLoS Comput Biol. 2021. PMID: 33647016 Free PMC article.
-
Induction and Maturation of Hepatocyte-Like Cells In Vitro: Focus on Technological Advances and Challenges.Front Cell Dev Biol. 2021 Nov 26;9:765980. doi: 10.3389/fcell.2021.765980. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901010 Free PMC article. Review.
-
Adaptation within embryonic and neonatal heart environment reveals alternative fates for adult c-kit+ cardiac interstitial cells.Stem Cells Transl Med. 2020 May;9(5):620-635. doi: 10.1002/sctm.19-0277. Epub 2019 Dec 31. Stem Cells Transl Med. 2020. PMID: 31891237 Free PMC article.
-
Human-Monkey Chimeras for Modeling Human Disease: Opportunities and Challenges.Stem Cells Dev. 2018 Dec 1;27(23):1599-1604. doi: 10.1089/scd.2018.0162. Stem Cells Dev. 2018. PMID: 30319057 Free PMC article. Review.
-
Feasibility of large experimental animal models in testing novel therapeutic strategies for diabetes.World J Diabetes. 2021 Apr 15;12(4):306-330. doi: 10.4239/wjd.v12.i4.306. World J Diabetes. 2021. PMID: 33889282 Free PMC article. Review.
References
-
- Yang L, Guell M, Niu D, George H, Lesha E, Grishin D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015;350:1101–4. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
