Structure-Function Relationships of Glycine and GABAA Receptors and Their Interplay With the Scaffolding Protein Gephyrin
- PMID: 30258351
- PMCID: PMC6143783
- DOI: 10.3389/fnmol.2018.00317
Structure-Function Relationships of Glycine and GABAA Receptors and Their Interplay With the Scaffolding Protein Gephyrin
Abstract
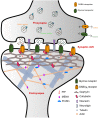
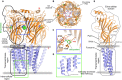
Glycine and γ-aminobutyric acid (GABA) are the major determinants of inhibition in the central nervous system (CNS). These neurotransmitters target glycine and GABAA receptors, respectively, which both belong to the Cys-loop superfamily of pentameric ligand-gated ion channels (pLGICs). Interactions of the neurotransmitters with the cognate receptors result in receptor opening and a subsequent influx of chloride ions, which, in turn, leads to hyperpolarization of the membrane potential, thus counteracting excitatory stimuli. The majority of glycine receptors and a significant fraction of GABAA receptors (GABAARs) are recruited and anchored to the post-synaptic membrane by the central scaffolding protein gephyrin. This ∼93 kDa moonlighting protein is structurally organized into an N-terminal G-domain (GephG) connected to a C-terminal E-domain (GephE) via a long unstructured linker. Both inhibitory neurotransmitter receptors interact via a short peptide motif located in the large cytoplasmic loop located in between transmembrane helices 3 and 4 (TM3-TM4) of the receptors with a universal receptor-binding epitope residing in GephE. Gephyrin engages in nearly identical interactions with the receptors at the N-terminal end of the peptide motif, and receptor-specific interaction toward the C-terminal region of the peptide. In addition to its receptor-anchoring function, gephyrin also interacts with a rather large collection of macromolecules including different cytoskeletal elements, thus acting as central scaffold at inhibitory post-synaptic specializations. Dysfunctions in receptor-mediated or gephyrin-mediated neurotransmission have been identified in various severe neurodevelopmental disorders. Although biochemical, cellular and electrophysiological studies have helped to understand the physiological and pharmacological roles of the receptors, recent high resolution structures of the receptors have strengthened our understanding of the receptors and their gating mechanisms. Besides that, multiple crystal structures of GephE in complex with receptor-derived peptides have shed light into receptor clustering by gephyrin at inhibitory post-synapses. This review will highlight recent biochemical and structural insights into gephyrin and the GlyRs as well as GABAA receptors, which provide a deeper understanding of the molecular machinery mediating inhibitory neurotransmission.
Keywords: GABAA receptors; cytoskeletal proteins; gephyrin; glycine receptors; inhibitory post-synaptic specialization; moonlighting protein.
Figures








References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

