The Role of Antibiotic-Target-Modifying and Antibiotic-Modifying Enzymes in Mycobacterium abscessus Drug Resistance
- PMID: 30258428
- PMCID: PMC6143652
- DOI: 10.3389/fmicb.2018.02179
The Role of Antibiotic-Target-Modifying and Antibiotic-Modifying Enzymes in Mycobacterium abscessus Drug Resistance
Abstract
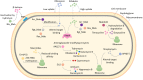
The incidence and prevalence of non-tuberculous mycobacterial (NTM) infections have been increasing worldwide and lately led to an emerging public health problem. Among rapidly growing NTM, Mycobacterium abscessus is the most pathogenic and drug resistant opportunistic germ, responsible for disease manifestations ranging from "curable" skin infections to only "manageable" pulmonary disease. Challenges in M. abscessus treatment stem from the bacteria's high-level innate resistance and comprise long, costly and non-standardized administration of antimicrobial agents, poor treatment outcomes often related to adverse effects and drug toxicities, and high relapse rates. Drug resistance in M. abscessus is conferred by an assortment of mechanisms. Clinically acquired drug resistance is normally conferred by mutations in the target genes. Intrinsic resistance is attributed to low permeability of M. abscessus cell envelope as well as to (multi)drug export systems. However, expression of numerous enzymes by M. abscessus, which can modify either the drug-target or the drug itself, is the key factor for the pathogen's phenomenal resistance to most classes of antibiotics used for treatment of other moderate to severe infectious diseases, like macrolides, aminoglycosides, rifamycins, β-lactams and tetracyclines. In 2009, when M. abscessus genome sequence became available, several research groups worldwide started studying M. abscessus antibiotic resistance mechanisms. At first, lack of tools for M. abscessus genetic manipulation severely delayed research endeavors. Nevertheless, the last 5 years, significant progress has been made towards the development of conditional expression and homologous recombination systems for M. abscessus. As a result of recent research efforts, an erythromycin ribosome methyltransferase, two aminoglycoside acetyltransferases, an aminoglycoside phosphotransferase, a rifamycin ADP-ribosyltransferase, a β-lactamase and a monooxygenase were identified to frame the complex and multifaceted intrinsic resistome of M. abscessus, which clearly contributes to complications in treatment of this highly resistant pathogen. Better knowledge of the underlying mechanisms of drug resistance in M. abscessus could improve selection of more effective chemotherapeutic regimen and promote development of novel antimicrobials which can overwhelm the existing resistance mechanisms. This article reviews the currently elucidated molecular mechanisms of antibiotic resistance in M. abscessus, with a focus on its drug-target-modifying and drug-modifying enzymes.
Keywords: Mycobacterium abscessus; antibiotic; antibiotic-modifying enzymes; antibiotic-target-modifying enzymes; drug resistance; non-tuberculous mycobacteria; resistance genes.
Figures
References
-
- Aínsa J. A., Pérez E., Pelicic V., Berthet F. X., Gicquel B., Martín C. (1997). Aminoglycoside 2′-N-acetyltransferase genes are universally present in mycobacteria: characterization of the aac(2′)-Ic gene from Mycobacterium tuberculosis and the aac(2’)-Id gene from Mycobacterium smegmatis. Mol. Microbiol. 24 431–441. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

