The Cellular DExD/H-Box RNA Helicase UAP56 Co-localizes With the Influenza A Virus NS1 Protein
- PMID: 30258431
- PMCID: PMC6144874
- DOI: 10.3389/fmicb.2018.02192
The Cellular DExD/H-Box RNA Helicase UAP56 Co-localizes With the Influenza A Virus NS1 Protein
Abstract
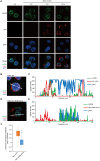
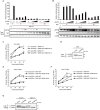
UAP56, a member of the DExD/H-box RNA helicase family, is essential for pre-mRNA splicing and mRNA export in eukaryotic cells. In influenza A virus-infected cells, UAP56 mediates viral mRNA nuclear export, facilitates viral ribonucleoprotein complex formation through direct interaction with the viral nucleoprotein, and may indirectly affect antiviral host responses by binding to and/or facilitating the activation of the antiviral host factors MxA and PKR. Here, we demonstrate that UAP56 also co-localizes with the influenza A viral NS1 protein, which counteracts host cell innate immune responses stimulated by virus infection. The UAP56-NS1 association relies on the RNA-binding residues R38 and K41 in NS1 and may be mediated by single-stranded RNA. UAP56 association with NS1 does not affect the NS1-mediated downregulation of cellular innate immune pathways in reporter gene assays, leaving in question the exact biological role and relevance of the UAP56-NS1 association.
Keywords: UAP56; host factors; influenza A NS1; influenza A virus; nuclear localization.
Figures



Similar articles
-
The cellular RNA helicase UAP56 is required for prevention of double-stranded RNA formation during influenza A virus infection.J Virol. 2011 Sep;85(17):8646-55. doi: 10.1128/JVI.02559-10. Epub 2011 Jun 15. J Virol. 2011. PMID: 21680511 Free PMC article.
-
Interferon-induced antiviral protein MxA interacts with the cellular RNA helicases UAP56 and URH49.J Biol Chem. 2011 Oct 7;286(40):34743-51. doi: 10.1074/jbc.M111.251843. Epub 2011 Aug 22. J Biol Chem. 2011. PMID: 21859714 Free PMC article.
-
Cellular UAP56 interacts with the HBx protein of the hepatitis B virus and is involved in viral RNA nuclear export in hepatocytes.Exp Cell Res. 2020 May 1;390(1):111929. doi: 10.1016/j.yexcr.2020.111929. Epub 2020 Mar 10. Exp Cell Res. 2020. PMID: 32169426
-
UAP56- a key player with surprisingly diverse roles in pre-mRNA splicing and nuclear export.BMB Rep. 2009 Apr 30;42(4):185-8. doi: 10.5483/bmbrep.2009.42.4.185. BMB Rep. 2009. PMID: 19403039 Review.
-
Critical Cellular Functions and Mechanisms of Action of the RNA Helicase UAP56.J Mol Biol. 2024 Jun 15;436(12):168604. doi: 10.1016/j.jmb.2024.168604. Epub 2024 May 8. J Mol Biol. 2024. PMID: 38729260 Free PMC article. Review.
Cited by
-
Viral modulation of cellular RNA alternative splicing: A new key player in virus-host interactions?Wiley Interdiscip Rev RNA. 2019 Sep;10(5):e1543. doi: 10.1002/wrna.1543. Epub 2019 Apr 29. Wiley Interdiscip Rev RNA. 2019. PMID: 31034770 Free PMC article. Review.
-
Variant- and vaccination-specific alternative splicing profiles in SARS-CoV-2 infections.iScience. 2024 Feb 8;27(3):109177. doi: 10.1016/j.isci.2024.109177. eCollection 2024 Mar 15. iScience. 2024. PMID: 38414855 Free PMC article.
-
Host Cell Factors That Interact with Influenza Virus Ribonucleoproteins.Cold Spring Harb Perspect Med. 2021 Nov 1;11(11):a038307. doi: 10.1101/cshperspect.a038307. Cold Spring Harb Perspect Med. 2021. PMID: 32988980 Free PMC article. Review.
-
Variant- and Vaccination-Specific Alternative Splicing Profiles in SARS-CoV-2 Infections.bioRxiv [Preprint]. 2023 Nov 27:2023.11.24.568603. doi: 10.1101/2023.11.24.568603. bioRxiv. 2023. Update in: iScience. 2024 Feb 08;27(3):109177. doi: 10.1016/j.isci.2024.109177. PMID: 38076812 Free PMC article. Updated. Preprint.
-
ILDR1 promotes influenza A virus replication through binding to PLSCR1.Sci Rep. 2022 May 20;12(1):8515. doi: 10.1038/s41598-022-12598-3. Sci Rep. 2022. PMID: 35595813 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

