Akt2 Regulates the Differentiation and Function of NKT17 Cells via FoxO-1-ICOS Axis
- PMID: 30258434
- PMCID: PMC6143662
- DOI: 10.3389/fimmu.2018.01940
Akt2 Regulates the Differentiation and Function of NKT17 Cells via FoxO-1-ICOS Axis
Erratum in
-
Corrigendum: Akt2 Regulates the Differentiation and Function of NKT17 Cells via FoxO-1-ICOS Axis.Front Immunol. 2020 Sep 30;11:589346. doi: 10.3389/fimmu.2020.589346. eCollection 2020. Front Immunol. 2020. Retraction in: Front Immunol. 2021 Jun 14;12:716458. doi: 10.3389/fimmu.2021.716458. PMID: 33101321 Free PMC article. Retracted.
Retraction in
-
Retraction: Akt2 Regulates the Differentiation and Function of NKT17 Cells via FoxO-1-ICOS Axis.Front Immunol. 2021 Mar 31;12:686304. doi: 10.3389/fimmu.2021.686304. eCollection 2021. Front Immunol. 2021. PMID: 33868320 Free PMC article.
Abstract
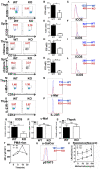
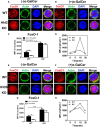
As a critical linker between mTORC1 and mTORC2, Akt is important for the cell metabolism. The role of Akt in the function and development of B and T cells is well characterized, however, the role of Akt for development and function of iNKT cells is unknown. iNKT cells bridge the adaptive and innate immunity, and in this study, we found that the differentiation of NKT17 cells and IL17 production of NKT17 cells were disrupted in Akt2 KO mice. ICOS has been demonstrated to be critical for the differentiation of NKT17 cells and we found that ICOS mRNA and protein expression was reduced in Akt2 KO iNKT cells. As a consequence, phosphorylation of FoxO-1 was downregulated in Akt2 KO thymocytes but the sequestration of FoxO-1 in the nucleus of Akt2 KO iNKT cells was increased. The negative feedback loop between ICOS and FoxO-1 has been demonstrated in CD4+T follicular helper cells. Therefore our study has revealed a new intracellular mechanism in which Akt2 regulates ICOS expression via FoxO-1 and this signaling axis regulates the differentiation and function of NKT17 cells. This study provides a new linker between cell metabolism and function of iNKT cells.
Keywords: Akt2; FoxO-1; ICOS; NKT17; iNKT.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

