Dietary Docosahexaenoic Acid Prevents Silica-Induced Development of Pulmonary Ectopic Germinal Centers and Glomerulonephritis in the Lupus-Prone NZBWF1 Mouse
- PMID: 30258439
- PMCID: PMC6143671
- DOI: 10.3389/fimmu.2018.02002
Dietary Docosahexaenoic Acid Prevents Silica-Induced Development of Pulmonary Ectopic Germinal Centers and Glomerulonephritis in the Lupus-Prone NZBWF1 Mouse
Abstract
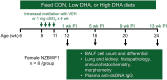
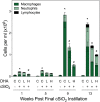
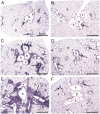
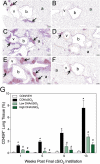
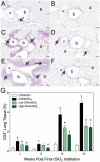
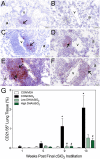
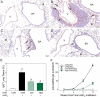
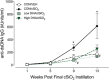
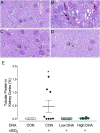
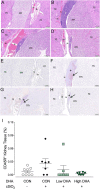
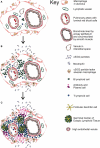
Ectopic lymphoid structures (ELS) consist of B-cell and T-cell aggregates that are initiated de novo in inflamed tissues outside of secondary lymphoid organs. When organized within follicular dendritic cell (FDC) networks, ELS contain functional germinal centers that can yield autoantibody-secreting plasma cells and promote autoimmune disease. Intranasal instillation of lupus-prone mice with crystalline silica (cSiO2), a respirable particle linked to human lupus, triggers ELS formation in the lung, systemic autoantibodies, and early onset of glomerulonephritis. Here we tested the hypothesis that consumption of docosahexaenoic acid (DHA), an ω-3 polyunsaturated fatty acid with anti-inflammatory properties, influences the temporal profile of cSiO2-induced pulmonary ectopic germinal center formation and development of glomerulonephritis. Female NZBWF1 mice (6-wk old) were fed purified isocaloric diets supplemented with 0, 4, or 10 g/kg DHA - calorically equivalent to 0, 2, or 5 g DHA per day consumption by humans, respectively. Beginning at age 8 wk, mice were intranasally instilled with 1 mg cSiO2, or saline vehicle alone, once per wk, for 4 wk. Cohorts were sacrificed 1, 5, 9, or 13 wk post-instillation (PI) of the last cSiO2 dose, and lung and kidney lesions were investigated by histopathology. Tissue fatty acid analyses confirmed uniform dose-dependent DHA incorporation across all cohorts. As early as 1 wk PI, inflammation comprising of B (CD45R+) and T (CD3+) cell accumulation was observed in lungs of cSiO2-treated mice compared to vehicle controls; these responses intensified over time. Marked follicular dendritic cell (FDC; CD21+/CD35+) networking appeared at 9 and 13 wk PI. IgG+ plasma cells suggestive of mature germinal centers were evident at 13 wk. DHA supplementation dramatically suppressed cSiO2-triggered B-cell, T-cell, FDC, and IgG+ plasma cell appearance in the lungs as well as anti-dsDNA IgG in bronchial lavage fluid and plasma over the course of the experiment. cSiO2 induced glomerulonephritis with concomitant B-cell accumulation in the renal cortex at 13 wk PI but this response was abrogated by DHA feeding. Taken together, realistic dietary DHA supplementation prevented initiation and/or progression of ectopic lymphoid neogenesis, germinal center development, systemic autoantibody elevation, and resultant glomerulonephritis in this unique preclinical model of environment-triggered lupus.
Keywords: autoimmunity; ectopic lymphoid structure; lung; omega-3; polyunsaturated fatty acid; silica; systemic lupus erythematosus.
Figures
Similar articles
-
Omega-3 Polyunsaturated Fatty Acid Intervention Against Established Autoimmunity in a Murine Model of Toxicant-Triggered Lupus.Front Immunol. 2021 Apr 7;12:653464. doi: 10.3389/fimmu.2021.653464. eCollection 2021. Front Immunol. 2021. PMID: 33897700 Free PMC article.
-
Omega-3 fatty acid intake suppresses induction of diverse autoantibody repertoire by crystalline silica in lupus-prone mice.Autoimmunity. 2020 Nov;53(7):415-433. doi: 10.1080/08916934.2020.1801651. Epub 2020 Sep 9. Autoimmunity. 2020. PMID: 32903098 Free PMC article.
-
Silica-Triggered Autoimmunity in Lupus-Prone Mice Blocked by Docosahexaenoic Acid Consumption.PLoS One. 2016 Aug 11;11(8):e0160622. doi: 10.1371/journal.pone.0160622. eCollection 2016. PLoS One. 2016. PMID: 27513935 Free PMC article.
-
Lupus, Silica, and Dietary Omega-3 Fatty Acid Interventions.Toxicol Pathol. 2019 Dec;47(8):1004-1011. doi: 10.1177/0192623319878398. Epub 2019 Nov 14. Toxicol Pathol. 2019. PMID: 31725357 Free PMC article. Review.
-
Centrality of Myeloid-Lineage Phagocytes in Particle-Triggered Inflammation and Autoimmunity.Front Toxicol. 2021 Nov 4;3:777768. doi: 10.3389/ftox.2021.777768. eCollection 2021. Front Toxicol. 2021. PMID: 35295146 Free PMC article. Review.
Cited by
-
Mine-site derived particulate matter exposure exacerbates neurological and pulmonary inflammatory outcomes in an autoimmune mouse model.J Toxicol Environ Health A. 2021 Jun 18;84(12):503-517. doi: 10.1080/15287394.2021.1891488. Epub 2021 Mar 7. J Toxicol Environ Health A. 2021. PMID: 33682625 Free PMC article.
-
Mapping of Dynamic Transcriptome Changes Associated With Silica-Triggered Autoimmune Pathogenesis in the Lupus-Prone NZBWF1 Mouse.Front Immunol. 2019 Mar 29;10:632. doi: 10.3389/fimmu.2019.00632. eCollection 2019. Front Immunol. 2019. PMID: 30984195 Free PMC article.
-
Serum fatty acid profiles in systemic lupus erythematosus and patient reported outcomes: The Michigan Lupus Epidemiology & Surveillance (MILES) Program.Front Immunol. 2024 Dec 18;15:1459297. doi: 10.3389/fimmu.2024.1459297. eCollection 2024. Front Immunol. 2024. PMID: 39744623 Free PMC article.
-
Sex differences in the inflammatory immune response to multi-walled carbon nanotubes and crystalline silica.Inhal Toxicol. 2019 Jun;31(7):285-297. doi: 10.1080/08958378.2019.1669743. Epub 2019 Sep 26. Inhal Toxicol. 2019. PMID: 31556754 Free PMC article.
-
FKN Facilitates HK-2 Cell EMT and Tubulointerstitial Lesions via the Wnt/β-Catenin Pathway in a Murine Model of Lupus Nephritis.Front Immunol. 2019 Apr 30;10:784. doi: 10.3389/fimmu.2019.00784. eCollection 2019. Front Immunol. 2019. PMID: 31134047 Free PMC article.
References
-
- Nacionales DC, Weinstein JS, Yan X-J, Albesiano E, Lee PY, Kelly-Scumpia KM, et al. . B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus. J Immunol. (2009) 182:4226–36. 10.4049/jimmunol.0800771 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

