IL-6 Receptor Inhibition by Tocilizumab Attenuated Expression of C5a Receptor 1 and 2 in Non-ST-Elevation Myocardial Infarction
- PMID: 30258440
- PMCID: PMC6143659
- DOI: 10.3389/fimmu.2018.02035
IL-6 Receptor Inhibition by Tocilizumab Attenuated Expression of C5a Receptor 1 and 2 in Non-ST-Elevation Myocardial Infarction
Abstract
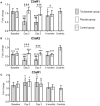
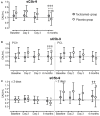
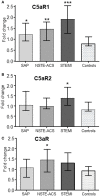
Background: Elevated interleukin-6 (IL-6) and complement activation are associated with detrimental effects of inflammation in coronary artery disease (CAD). The complement anaphylatoxins C5a and C3a interact with their receptors; the highly inflammatory C5aR1, and the C5aR2 and C3aR. We evaluated the effect of the IL-6 receptor (IL-6R)-antagonist tocilizumab on the expression of the anaphylatoxin receptors in whole blood from non-ST-elevation myocardial infarction (NSTEMI) patients. Separately, anaphylatoxin receptor expression in peripheral blood mononuclear cells (PBMC) from patients with different entities of CAD was investigated. Materials and Methods: NSTEMI patients were randomized to one dose of tocilizumab (n = 28) or placebo (n = 32) and observed for 6 months. Whole blood samples drawn at inclusion, at day 2, 3 and after 6 months were used for mRNA isolation. Plasma was prepared for analysis of complement activation measured as sC5b-9 by ELISA. Furthermore, patients with different CAD entities comprising stable angina pectoris (SAP, n = 22), non-ST-elevation acute coronary syndrome (NSTE-ACS, n = 21) and ST-elevation myocardial infarction (STEMI, n = 20) were included. PBMC was isolated from blood samples obtained at admission to hospital and mRNA isolated. Anaphylatoxin-receptor-expression was analyzed with qPCR using mRNA from whole blood and PBMC, respectively. Results: Our main findings were (i) Tocilizumab decreased C5aR1 and C5aR2 mRNA expression significantly (p < 0.001) and substantially (>50%) at day 2 and 3, whereas C3aR expression was unaffected. (ii) Tocilizumab did not affect complement activation. (iii) In analyzes of different CAD entities, C5aR1 expression was significantly increased in all CAD subgroups compared to controls with the highest level in the STEMI patients (p < 0.001). For C5aR2 and C3aR the expression compared to controls were more moderate with increased expression of C5aR2 in the STEMI group (p < 0.05) and C3aR in the NSTE-ACS group (p < 0.05). Conclusion: Expression of C5aR1 and C5aR2 in whole blood was significantly attenuated by IL-6R-inhibition in NSTEMI patients. These receptors were significantly upregulated in PBMC CAD patients with particularly high levels of C5aR1 in STEMI patients.
Keywords: C3a receptor; C5a receptors; IL-6; complement; inflammation; myocardial infarction.
Figures

References
-
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:267–315. 10.1093/eurheartj/ehv320 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

