Activation of RIG-I-Mediated Antiviral Signaling Triggers Autophagy Through the MAVS-TRAF6-Beclin-1 Signaling Axis
- PMID: 30258449
- PMCID: PMC6143786
- DOI: 10.3389/fimmu.2018.02096
Activation of RIG-I-Mediated Antiviral Signaling Triggers Autophagy Through the MAVS-TRAF6-Beclin-1 Signaling Axis
Abstract
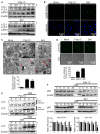
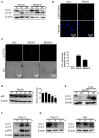
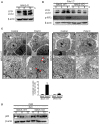
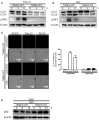
Autophagy has been implicated in innate immune responses against various intracellular pathogens. Recent studies have reported that autophagy can be triggered by pathogen recognizing sensors, including Toll-like receptors and cyclic guanosine monophosphate-adenosine monophosphate synthase, to participate in innate immunity. In the present study, we examined whether the RIG-I signaling pathway, which detects viral infections by recognizing viral RNA, triggers the autophagic process. The introduction of polyI:C into the cytoplasm, or Sendai virus infection, significantly induced autophagy in normal cells but not in RIG-I-deficient cells. PolyI:C transfection or Sendai virus infection induced autophagy in the cells lacking type-I interferon signaling. This demonstrated that the effect was not due to interferon signaling. RIG-I-mediated autophagy diminished by the deficiency of mitochondrial antiviral signaling protein (MAVS) or tumor necrosis factor receptor-associated factor (TRAF)6, showing that the RIG-I-MAVS-TRAF6 signaling axis was critical for RIG-I-mediated autophagy. We also found that Beclin-1 was translocated to the mitochondria, and it interacted with TRAF6 upon RIG-I activation. Furthermore, Beclin-1 underwent K63-polyubiquitination upon RIG-I activation, and the ubiquitination decreased in TRAF6-deficient cells. This suggests that the RIG-I-MAVS-TRAF6 axis induced K63-linked polyubiquitination of Beclin-1, which has been implicated in triggering autophagy. As deficient autophagy increases the type-I interferon response, the induction of autophagy by the RIG-I pathway might also contribute to preventing an excessive interferon response as a negative-feedback mechanism.
Keywords: Beclin-1; MAVS; RIG-I; TRAF6; autophagy; innate immunity; polyubiquitination.
Figures



Similar articles
-
Inhibition of the RLR signaling pathway by SARS-CoV-2 ORF7b is mediated by MAVS and abrogated by ORF7b-homologous interfering peptide.J Virol. 2024 May 14;98(5):e0157323. doi: 10.1128/jvi.01573-23. Epub 2024 Apr 4. J Virol. 2024. PMID: 38572974 Free PMC article.
-
Influenza M2 protein regulates MAVS-mediated signaling pathway through interacting with MAVS and increasing ROS production.Autophagy. 2019 Jul;15(7):1163-1181. doi: 10.1080/15548627.2019.1580089. Epub 2019 Feb 20. Autophagy. 2019. PMID: 30741586 Free PMC article.
-
Spatiotemporal dynamics of innate immune signaling via RIG-I-like receptors.Proc Natl Acad Sci U S A. 2020 Jul 7;117(27):15778-15788. doi: 10.1073/pnas.1921861117. Epub 2020 Jun 22. Proc Natl Acad Sci U S A. 2020. PMID: 32571931 Free PMC article.
-
Regulation of MAVS Expression and Signaling Function in the Antiviral Innate Immune Response.Front Immunol. 2020 May 27;11:1030. doi: 10.3389/fimmu.2020.01030. eCollection 2020. Front Immunol. 2020. PMID: 32536927 Free PMC article. Review.
-
Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses.Front Immunol. 2020 Jun 24;11:1296. doi: 10.3389/fimmu.2020.01296. eCollection 2020. Front Immunol. 2020. PMID: 32670286 Free PMC article. Review.
Cited by
-
Death domain fold proteins in immune signaling and transcriptional regulation.FEBS J. 2022 Jul;289(14):4082-4097. doi: 10.1111/febs.15901. Epub 2021 May 13. FEBS J. 2022. PMID: 33905163 Free PMC article. Review.
-
Typically inhibiting USP14 promotes autophagy in M1-like macrophages and alleviates CLP-induced sepsis.Cell Death Dis. 2020 Aug 20;11(8):666. doi: 10.1038/s41419-020-02898-9. Cell Death Dis. 2020. PMID: 32820146 Free PMC article.
-
Supramolecular Complexes in Cell Death and Inflammation and Their Regulation by Autophagy.Front Cell Dev Biol. 2019 May 3;7:73. doi: 10.3389/fcell.2019.00073. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31131275 Free PMC article. Review.
-
SARS-CoV-2 infection-induced immune responses: Friends or foes?Scand J Immunol. 2020 Aug;92(2):e12895. doi: 10.1111/sji.12895. Epub 2020 Jun 17. Scand J Immunol. 2020. PMID: 32445403 Free PMC article. Review.
-
Germline Genetic Variants of Viral Entry and Innate Immunity May Influence Susceptibility to SARS-CoV-2 Infection: Toward a Polygenic Risk Score for Risk Stratification.Front Immunol. 2021 Mar 8;12:653489. doi: 10.3389/fimmu.2021.653489. eCollection 2021. Front Immunol. 2021. PMID: 33763088 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

