A Study of Cutaneous Adverse Drug Reactions in a Tertiary Care Center in Punjab
- PMID: 30258795
- PMCID: PMC6137653
- DOI: 10.4103/idoj.IDOJ_81_18
A Study of Cutaneous Adverse Drug Reactions in a Tertiary Care Center in Punjab
Abstract
Context: Cutaneous adverse drug eruptions are the most common adverse reactions attributed to drugs in which any type of skin reaction can be mimicked, induced, or aggravated.
Aims: To study the pattern of various types of cutaneous adverse drug reactions (CADRs), to find out the causative drug(s) involved and to determine the response to treatment and outcome in patients with CADRs.
Patients and methods: This prospective study was done in the department of dermatology. Patients with suspected drug rash, of either sex and all age groups were included in the study.
Statistical analysis: Frequencies and proportions were calculated using Chi-square test and t-test as the tests of significance. Data was analyzed using SPSS version 21.
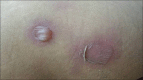
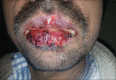
Results: A total of 258 patients were enrolled in the study. The most common CADR observed in the study was exanthematous drug eruption in 42.63% patients followed by drug induced urticaria in 21.32% patients. Antimicrobials were the most common offending drugs in 64.73% of patients, followed by non-steroidal anti-inflammatory drugs (NSAIDs) in 15.50% patients. In the study, 12 patients (4.65%) were found to have severe cutaneous adverse drug reactions (SCADRs). Stevens-Johnson syndrome (SJS) - Toxic epidermal necrolysis (TEN) was the most common SCADR (50%) and antituberculous drugs were the most common causative group of drugs causing SCADRs.
Conclusion: The most common CADR observed in the study was exanthematous drug eruption and antimicrobials were the most common causative drugs.
Keywords: Antimicrobials; cutaneous adverse drug reactions; exanthematous drug eruption.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Bhatia A, Kanish B, Ziauddin M, Badyal D, Kartik Cutaneous adverse drug reaction monitoring in a tertiary care teaching hospital. Clin Res. 2013;5:60–5.
-
- Choon S, Lai N. An epidemiological and clinical analysis of cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia. Indian J Dermatol Venereol Leprol. 2012;78:734–9. - PubMed
-
- Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India. Indian J Dermatol Venereol Leprol. 2004;70:20–4. - PubMed