P53 rs1042522 and CD95 rs1800682 genetic variations in HCV-4a response to antiviral therapy
- PMID: 30258864
- PMCID: PMC6150111
- DOI: 10.1016/j.gendis.2015.02.004
P53 rs1042522 and CD95 rs1800682 genetic variations in HCV-4a response to antiviral therapy
Abstract
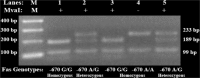
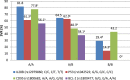
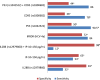
Current estimates indicate that the hepatitis C (HCV) is the leading cause of mortality around the world, with infection rates steadily increasing in Egypt. The dual therapy for this silent epidemic with pegylated-interferon-α2b/ribavirin has markedly improved the success rates in genotype-4 patients. It was reported that apoptosis plays a vital mechanistic role in limiting viral replication. P53, a key regulator of apoptosis, induces CD95 gene expression and subsequently initiates apoptotic cascade to be activated. The current study examined the impact of P53 rs1042522 and CD95 rs1800682 polymorphisms on the treatment response. Three groups of 240 volunteers were enrolled in this study; 86 in sustained virological responders group, 74 in non-responders group, and 80 in control group. All patients had HCV genotype-4a and were interferon treatment naïve. Quantizations of HCV-RNA by qRT-PCR and histological scores were performed for all patients. In addition, genotyping of HCV-RNA, P53 rs1042522 Arg/Pro and CD95 rs1800682 A/G polymorphisms were investigated in all subjects. It was resulted that P53 Pro/Pro homozygous genotype has high significant increase, while CD95 A/A homozygous genotype has high significant decrease when comparing non-responders with responders. Finally, it was concluded that Pro variant of P53 rs1042522 may be used as a genetic predictor for non-responsiveness, while A/A variant of CD95 rs1800682 may be used as a sensitive biomarker for responsiveness to antiviral therapy of HCV genotype-4a infection. In addition, low prolactin, high total testosterone, and high GH levels may provide promising biomarkers for early prediction of the response when associated with these genetic polymorphisms.
Keywords: CD95 rs1800682; HCV gentotype-4a; P53 rs1042522; Response; SNPs.
Figures


Similar articles
-
The potential impact of P53 and APO-1 genetic polymorphisms on hepatitis C genotype 4a susceptibility.Gene. 2014 Oct 15;550(1):40-5. doi: 10.1016/j.gene.2014.08.011. Epub 2014 Aug 7. Gene. 2014. PMID: 25108128
-
Effectiveness of Sofosbuvir, Ribavirin and PEG-IFNα-2a in the Treatment of Naïve Egyptian Patients With Chronic Hepatitis C Virus Genotype 4.Am J Med Sci. 2018 May;355(5):456-466. doi: 10.1016/j.amjms.2017.12.016. Epub 2017 Dec 29. Am J Med Sci. 2018. PMID: 29753376
-
Gene polymorphisms of IL-10 and MxA in responders and non-responders to interferon therapy in HCV Egyptian patients genotype 4.Cell Biochem Biophys. 2015 Mar;71(2):617-25. doi: 10.1007/s12013-014-0241-9. Cell Biochem Biophys. 2015. PMID: 25239021
-
Current progress in the treatment of chronic hepatitis C.World J Gastroenterol. 2012 Nov 14;18(42):6060-9. doi: 10.3748/wjg.v18.i42.6060. World J Gastroenterol. 2012. PMID: 23155334 Free PMC article. Review.
-
Antiviral therapies for chronic hepatitis C virus infection with cirrhosis.World J Hepatol. 2015 May 18;7(8):1133-41. doi: 10.4254/wjh.v7.i8.1133. World J Hepatol. 2015. PMID: 26052402 Free PMC article. Review.
References
-
- Choo Q.L., Weiner A.J., Overby L.R., Bradley D.W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. - PubMed
-
- Mohd Hanafiah K., Groeger J., Flaxman A.D., Wiersma S.T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. J Hepatol. 2013;57:1333–1342. - PubMed
-
- Belousova V., Abd-Rabou A.A., Mousa S.A. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92–102. - PubMed
-
- U.S. Food and Drug Administration Approves Gilead's Sovaldi™ (Sofosbuvir) for the Treatment of Chronic Hepatitis C [press release] 2013.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

