Characterization of the essential role of bone morphogenetic protein 9 (BMP9) in osteogenic differentiation of mesenchymal stem cells (MSCs) through RNA interference
- PMID: 30258947
- PMCID: PMC6149187
- DOI: 10.1016/j.gendis.2018.04.006
Characterization of the essential role of bone morphogenetic protein 9 (BMP9) in osteogenic differentiation of mesenchymal stem cells (MSCs) through RNA interference
Erratum in
-
Corrigendum to "Characterization of the essential role of bone morphogenetic protein 9 (BMP9) in osteogenic differentiation of mesenchymal stem cells (MSCs) through RNA interference" [Genes & Diseases 5(2018):172-184].Genes Dis. 2023 Mar 23;10(2):632-637. doi: 10.1016/j.gendis.2023.03.001. eCollection 2023 Mar. Genes Dis. 2023. PMID: 37223525 Free PMC article.
Abstract
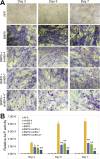
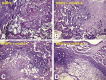
Mesenchymal stem cells (MSCs) are multipotent stem cells and capable of differentiating into multiple cell types including osteoblastic, chondrogenic and adipogenic lineages. We previously identified BMP9 as one of the most potent BMPs that induce osteoblastic differentiation of MSCs although exact molecular mechanism through which BMP9 regulates osteogenic differentiation remains to be fully understood. Here, we seek to develop a recombinant adenovirus system to optimally silence mouse BMP9 and then characterize the important role of BMP9 in osteogenic differentiation of MSCs. Using two different siRNA bioinformatic prediction programs, we design five siRNAs targeting mouse BMP9 (or simB9), which are expressed under the control of the converging H1 and U6 promoters in recombinant adenovirus vectors. We demonstrate that two of the five siRNAs, simB9-4 and simB9-7, exhibit the highest efficiency on silencing exogenous mouse BMP9 in MSCs. Furthermore, simB9-4 and simB9-7 act synergistically in inhibiting BMP9-induced expression of osteogenic markers, matrix mineralization and ectopic bone formation from MSCs. Thus, our findings demonstrate the important role of BMP9 in osteogenic differentiation of MSCs. The characterized simB9 siRNAs may be used as an important tool to investigate the molecular mechanism behind BMP9 osteogenic signaling. Our results also indicate that recombinant adenovirus-mediated expression of siRNAs is efficient and sustained, and thus may be used as an effective delivery vehicle of siRNA therapeutics.
Keywords: BMP9; Bone formation; Mesenchymal stem cells; Osteogenic differentiation; RNA interference; Recombinant adenovirus; siRNA.
Figures





References
-
- Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. - PubMed
-
- Caplan A.I., Bruder S.P. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–264. - PubMed
-
- Deng Z.L., Sharff K.A., Tang N. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

