Media studies to enhance the production of verticillins facilitated by in situ chemical analysis
- PMID: 30259213
- PMCID: PMC6251749
- DOI: 10.1007/s10295-018-2083-8
Media studies to enhance the production of verticillins facilitated by in situ chemical analysis
Abstract
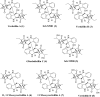
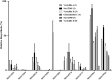
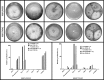
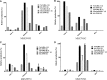
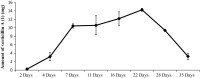
Verticillins are a group of epipolythiodioxopiperazine alkaloids that have displayed potent cytotoxicity. To evaluate their potential further, a larger supply of these compounds was needed for both in vivo studies and analogue development via semisynthesis. To optimize the biosynthesis of these secondary metabolites, their production was analyzed in two different fungal strains (MSX59553 and MSX79542) under a suite of fermentation conditions. These studies were facilitated by the use of the droplet-liquid microjunction-surface sampling probe (droplet probe), which enables chemical analysis in situ directly from the surface of the cultures. These experiments showed that the production of verticillins was greatly affected by growth conditions; a significantly higher quantity of these alkaloids was noted when the fungal strains were grown on an oatmeal-based medium. Using these technologies to select the best among the tested growth conditions, the production of the verticillin analogues was increased while concomitantly decreasing the time required for fermentations from 5 weeks to about 11 days. Importantly, where we could previously supply 5-10 mg every 6 weeks, we are now able to supply 50-150 mg quantities of key analogues per month via laboratory scale fermentation.
Keywords: Epipolythiodioxopiperazine alkaloids; Fermentation; Filamentous fungi; In situ extraction; Verticillin.
Figures

Similar articles
-
Exploration of Verticillins in High-Grade Serous Ovarian Cancer and Evaluation of Multiple Formulations in Preclinical In Vitro and In Vivo Models.Mol Pharm. 2023 Jun 5;20(6):3049-3059. doi: 10.1021/acs.molpharmaceut.3c00069. Epub 2023 May 8. Mol Pharm. 2023. PMID: 37155928 Free PMC article.
-
Engineering Fluorine into Verticillins (Epipolythiodioxopiperazine Alkaloids) via Precursor-Directed Biosynthesis.J Nat Prod. 2019 Nov 22;82(11):3104-3110. doi: 10.1021/acs.jnatprod.9b00711. Epub 2019 Oct 21. J Nat Prod. 2019. PMID: 31633350 Free PMC article.
-
Optimization of culture conditions for penicilazaphilone C production by a marine-derived fungus Penicillium sclerotiorum M-22.Lett Appl Microbiol. 2018 Mar;66(3):222-230. doi: 10.1111/lam.12841. Epub 2018 Feb 7. Lett Appl Microbiol. 2018. PMID: 29285768
-
Plasma pharmacokinetics and bioavailability of verticillin A following different routes of administration in mice using liquid chromatography tandem mass spectrometry.J Pharm Biomed Anal. 2017 May 30;139:187-192. doi: 10.1016/j.jpba.2017.02.051. Epub 2017 Mar 1. J Pharm Biomed Anal. 2017. PMID: 28284083 Free PMC article.
-
Fungal Morphology in Industrial Enzyme Production--Modelling and Monitoring.Adv Biochem Eng Biotechnol. 2015;149:29-54. doi: 10.1007/10_2015_309. Adv Biochem Eng Biotechnol. 2015. PMID: 25724310 Review.
Cited by
-
H3K9me3 represses G6PD expression to suppress the pentose phosphate pathway and ROS production to promote human mesothelioma growth.Oncogene. 2022 Apr;41(18):2651-2662. doi: 10.1038/s41388-022-02283-0. Epub 2022 Mar 30. Oncogene. 2022. PMID: 35351997 Free PMC article.
-
Discovery of Anticancer Agents of Diverse Natural Origin.J Nat Prod. 2022 Mar 25;85(3):702-719. doi: 10.1021/acs.jnatprod.2c00036. Epub 2022 Feb 25. J Nat Prod. 2022. PMID: 35213158 Free PMC article.
-
Exploration of Verticillins in High-Grade Serous Ovarian Cancer and Evaluation of Multiple Formulations in Preclinical In Vitro and In Vivo Models.Mol Pharm. 2023 Jun 5;20(6):3049-3059. doi: 10.1021/acs.molpharmaceut.3c00069. Epub 2023 May 8. Mol Pharm. 2023. PMID: 37155928 Free PMC article.
-
2,5-Diketopiperazines (DKPs): Promising Scaffolds for Anticancer Agents.Curr Pharm Des. 2024;30(8):597-623. doi: 10.2174/0113816128291798240201112916. Curr Pharm Des. 2024. PMID: 38343054 Review.
-
Semisynthetic Derivatives of the Verticillin Class of Natural Products through Acylation of the C11 Hydroxy Group.ACS Med Chem Lett. 2021 Mar 19;12(4):625-630. doi: 10.1021/acsmedchemlett.1c00024. eCollection 2021 Apr 8. ACS Med Chem Lett. 2021. PMID: 33859802 Free PMC article.
References
-
- Barrios-González J, Rodríguez GM, Tomasini A. Environmental and nutritional factors controlling aflatoxin production in cassava solid state fermentation. J Ferment Bioeng. 1990;70:329–333. doi: 10.1016/0922-338X(90)90144-L. - DOI
-
- Bennett JW, Ciegler A. Secondary metabolism and differentiation in fungi. New York: M. Dekker; 1983.
-
- Bills GF, Dombrowski AW, Goetz MA. The “FERMEX” method for metabolite-enriched fungal extracts. In: Keller NP, Turner G, editors. Fungal secondary metabolism: methods and protocols. New Jersey: Humana Press; 2012. pp. 79–96. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

