PACAP/PAC1 Expression and Function in Micturition Pathways
- PMID: 30259317
- PMCID: PMC6437024
- DOI: 10.1007/s12031-018-1170-7
PACAP/PAC1 Expression and Function in Micturition Pathways
Abstract
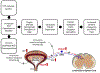
Neural injury, inflammation, or diseases commonly and adversely affect micturition reflex function that is organized by neural circuits in the CNS and PNS. One neuropeptide receptor system, pituitary adenylate cyclase-activating polypeptide (PACAP; Adcyap1), and its cognate receptor, PAC1 (Adcyap1r1), have tissue-specific distributions in the lower urinary tract. PACAP and associated receptors are expressed in the LUT and exhibit changes in expression, distribution, and function in preclinical animal models of bladder pain syndrome (BPS)/interstitial cystitis (IC), a chronic, visceral pain syndrome characterized by pain, and LUT dysfunction. Blockade of the PACAP/PAC1 receptor system reduces voiding frequency and somatic (e.g., hindpaw, pelvic) sensitivity in preclinical animal models and a transgenic mouse model that mirrors some clinical symptoms of BPS/IC. The PACAP/receptor system in micturition pathways may represent a potential target for therapeutic intervention to reduce LUT dysfunction following urinary bladder inflammation.
Keywords: Cyclophosphamide; Cystometry; NGF; Neuropeptides; Somatic sensation.
Conflict of interest statement
Conflicts of Interest
The authors declare that the research described from the Vizzard laboratory were conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aloe L, Tuveri MA, Carcassi U, and Levi-Montalcini R (1992). Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum 35(3), 351–355. - PubMed
-
- Andersson KE (2002). Bladder activation: afferent mechanisms. Urology 59(5 Suppl 1), 43–50. - PubMed
-
- Arimura A (1998). Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48(5), 301–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

