Cardiovascular Risk Management and Hepatitis C: Combining Drugs
- PMID: 30259390
- PMCID: PMC6451722
- DOI: 10.1007/s40262-018-0710-1
Cardiovascular Risk Management and Hepatitis C: Combining Drugs
Abstract
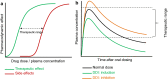
Direct-acting antivirals (DAAs) are known victims (substrate) and perpetrators (cause) of drug-drug interactions (DDIs). These DAAs are used for the treatment of hepatitis C virus (HCV) infections and are highly effective drugs. Drugs used for cardiovascular risk management are frequently used by HCV-infected patients, whom also are treated with DAAs. Therefore, the aim of this review was to describe DDIs between cardiovascular drugs (CVDs) and DAAs. An extensive literature search was performed containing search terms for the marketed DAAs and CVDs (β-blocking agents, ACE inhibitors, angiotensin II antagonists, renin inhibitors, diuretics, calcium channel blockers, statins/ezetimibe, fibrates, platelet aggregation inhibitors, vitamin K antagonists, heparins, direct Xa inhibitors, nitrates, amiodarone, and digoxin). In particular, the drug labels from the European Medicines Agency and the US Food and Drug Administration were used. A main finding of this review is that CVDs are mostly victims of DDIs with DAAs. Therefore, when possible, monitoring of pharmacodynamics is recommended when coadministering these drugs with DAAs. Nevertheless, it is sometimes better to discontinue a drug on a temporary basis (statins, ezetimide). The DAAs are victims of DDIs in combination with bisoprolol, carvedilol, labetalol, verapamil, and gemfibrozil. Despite there are many DDIs predicted in this review, most of these DDIs can be managed by monitoring the efficacy and toxicity of the victim drug or by switching to another CVD/DAA.
Conflict of interest statement
EJS declares having received travels grants from Abbvie and Gilead. SW and PJGTH declare that they have no conflicts of interest that are directly relevant to the content of this review. DMB is on the advisory boards for AbbVie, BMS, Gilead, Janssen, and Merck and received sponsorship/research grants from BMS, Gilead, Janssen, Merck, and Viiv. However, these conflicts of interest did not influence the preparation of this review.
Figures
References
-
- Lauffenburger JC, Mayer CL, Hawke RL, Brouwer KLR, Fried MW, Farley JF. Medication use and medical comorbidity in patients with chronic hepatitis C from a US commercial claims database: high utilization of drugs with interaction potential. Eur J Gastroenterol Hepatol. 2014;2014(26):1073–1082. doi: 10.1097/MEG.0000000000000152. - DOI - PMC - PubMed
-
- Vermehren J, Peiffer KH, Welsch C, Grammatikos G, Welker MW, Weiler N, et al. The efficacy and safety of direct acting antiviral treatment and clinical significance of drug-drug interactions in elderly patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2016;44(8):856–865. doi: 10.1111/apt.13769. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

