Chemokine (C-X-C motif) ligand 1 and CXCL2 produced by tumor promote the generation of monocytic myeloid-derived suppressor cells
- PMID: 30259595
- PMCID: PMC6272093
- DOI: 10.1111/cas.13809
Chemokine (C-X-C motif) ligand 1 and CXCL2 produced by tumor promote the generation of monocytic myeloid-derived suppressor cells
Abstract
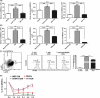
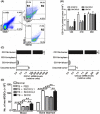
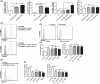
Accumulation of myeloid-derived suppressor cells (MDSC) in tumor-bearing hosts is a hallmark of tumor-associated inflammation, which is thought to be a barrier to immunosurveillance. Multiple factors secreted by tumor cells and tumor stromal cells are reported to be involved in promoting the expansion of MDSC. Herein, we showed that s.c. inoculation of tumor cells and i.v. injection of tumor-conditioned medium increased the number of MDSC. Subsequent investigation elucidated that chemokine (C-X-C motif) ligand 1 (CXCL1) and CXCL2, which were originally characterized as the chemokines of neutrophils, specifically promoted the expansion of monocytic MDSC (mo-MDSC), a subtype of MDSC, in the presence of granulocyte-macrophage colony-stimulating factor. Depletion of CXCL1 or CXCL2 in B16F10 cells or in B16F10-bearing mice noticeably decreased the generation of mo-MDSC in bone marrow. Moreover, we found that, in addition to the tumor cells, tumor-infiltrated CD11b+ myeloid cells also expressed CXCL1 and CXCL2. Furthermore, CXCL1- and CXCL2-induced increase of mo-MDSC was not correlated with chemotaxis, proliferation or apoptosis of mo-MDSC. These findings show a novel role of CXCL1 and CXCL2 in promoting mo-MDSC generation by favoring the differentiation of bone marrow cells in tumor-bearing conditions, which suggests that inhibition of CXCL1 and CXCL2 could decrease mo-MDSC generation and improve host immunosurveillance.
Keywords: CD11b+ myeloid cell; CXCL1; CXCL2; differentiation; mo-MDSC.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



Similar articles
-
Receptor-Interacting Protein Kinase 3 Deficiency Recruits Myeloid-Derived Suppressor Cells to Hepatocellular Carcinoma Through the Chemokine (C-X-C Motif) Ligand 1-Chemokine (C-X-C Motif) Receptor 2 Axis.Hepatology. 2019 Nov;70(5):1564-1581. doi: 10.1002/hep.30676. Epub 2019 Jul 17. Hepatology. 2019. PMID: 31021443 Free PMC article.
-
The Gustatory Sensory G-Protein GNAT3 Suppresses Pancreatic Cancer Progression in Mice.Cell Mol Gastroenterol Hepatol. 2021;11(2):349-369. doi: 10.1016/j.jcmgh.2020.08.011. Epub 2020 Aug 31. Cell Mol Gastroenterol Hepatol. 2021. PMID: 32882403 Free PMC article.
-
Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer.Clin Exp Metastasis. 2015 Apr;32(4):353-68. doi: 10.1007/s10585-015-9714-5. Epub 2015 Mar 24. Clin Exp Metastasis. 2015. PMID: 25802102 Free PMC article.
-
The Development and Homing of Myeloid-Derived Suppressor Cells: From a Two-Stage Model to a Multistep Narrative.Front Immunol. 2020 Oct 26;11:557586. doi: 10.3389/fimmu.2020.557586. eCollection 2020. Front Immunol. 2020. PMID: 33193327 Free PMC article. Review.
-
The role of CCR5 in directing the mobilization and biological function of CD11b+Gr1+Ly6Clow polymorphonuclear myeloid cells in cancer.Cancer Immunol Immunother. 2018 Dec;67(12):1949-1953. doi: 10.1007/s00262-018-2245-6. Epub 2018 Sep 19. Cancer Immunol Immunother. 2018. PMID: 30232521 Free PMC article. Review.
Cited by
-
CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer.Int J Mol Sci. 2022 Feb 16;23(4):2168. doi: 10.3390/ijms23042168. Int J Mol Sci. 2022. PMID: 35216283 Free PMC article. Review.
-
Splenectomy and azygoportal disconnection decreases the risk of hepatocellular carcinoma for cirrhosis patients with portal hypertension bleeding: a 10-year retrospective follow-up study based on the inverse probability of treatment weighting method.J Gastroenterol. 2023 May;58(5):503-512. doi: 10.1007/s00535-023-01982-z. Epub 2023 Mar 21. J Gastroenterol. 2023. PMID: 36943530
-
CNS tumor stroma transcriptomics identify perivascular fibroblasts as predictors of immunotherapy resistance in glioblastoma patients.NPJ Genom Med. 2023 Oct 26;8(1):35. doi: 10.1038/s41525-023-00381-w. NPJ Genom Med. 2023. PMID: 37884531 Free PMC article.
-
CCL18 in the Progression of Cancer.Int J Mol Sci. 2020 Oct 26;21(21):7955. doi: 10.3390/ijms21217955. Int J Mol Sci. 2020. PMID: 33114763 Free PMC article. Review.
-
Myeloid-derived suppressor cells attenuate the antitumor efficacy of radiopharmaceutical therapy using 90Y-NM600 in combination with androgen deprivation therapy in murine prostate tumors.J Immunother Cancer. 2024 Apr 24;12(4):e008760. doi: 10.1136/jitc-2023-008760. J Immunother Cancer. 2024. PMID: 38663936 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

