Plant responses underlying nonhost resistance of Citrus limon against Xanthomonas campestris pv. campestris
- PMID: 30260546
- PMCID: PMC6637874
- DOI: 10.1111/mpp.12752
Plant responses underlying nonhost resistance of Citrus limon against Xanthomonas campestris pv. campestris
Abstract
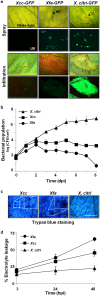
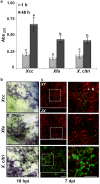
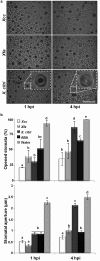
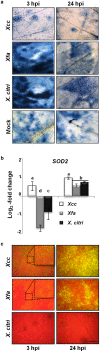
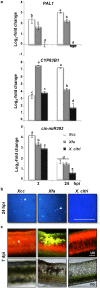
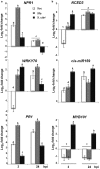
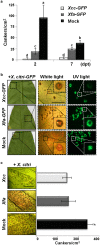
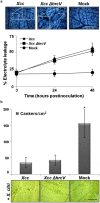
Citrus is an economically important fruit crop that is severely afflicted by citrus canker, a disease caused by Xanthomonas citri ssp. citri (X. citri); thus, new sustainable strategies to manage this disease are needed. Although all Citrus spp. are susceptible to this pathogen, they are resistant to other Xanthomonas species, exhibiting non-host resistance (NHR), for example, to the brassica pathogen X. campestris pv. campestris (Xcc) and a gene-for-gene host defence response (HDR) to the canker-causing X. fuscans ssp. aurantifolii (Xfa) strain C. Here, we examine the plant factors associated with the NHR of C. limon to Xcc. We show that Xcc induced asymptomatic type I NHR, allowing the bacterium to survive in a stationary phase in the non-host tissue. In C. limon, this NHR shared some similarities with HDR; both defence responses interfered with biofilm formation, and were associated with callose deposition, induction of the salicylic acid (SA) signalling pathway and the repression of abscisic acid (ABA) signalling. However, greater stomatal closure was seen during NHR than during HDR, together with different patterns of accumulation of reactive oxygen species and phenolic compounds and the expression of secondary metabolites. Overall, these differences, independent of Xcc type III effector proteins, could contribute to the higher protection elicited against canker development. We propose that Xcc may have the potential to steadily activate inducible defence responses. An understanding of these plant responses (and their triggers) may allow the development of a sustained and sustainable resistance to citrus canker.
Keywords: PAMP-triggered immunity; biofilm formation; canker disease; glucosinolates; protection; salicylic acid; stomatal immunity.
© 2018 BSPP and John Wiley & Sons Ltd.
Figures
Similar articles
-
Responsiveness of different citrus genotypes to the Xanthomonas citri ssp. citri-derived pathogen-associated molecular pattern (PAMP) flg22 correlates with resistance to citrus canker.Mol Plant Pathol. 2015 Jun;16(5):507-20. doi: 10.1111/mpp.12206. Epub 2014 Oct 27. Mol Plant Pathol. 2015. PMID: 25231217 Free PMC article.
-
Reclassification of Xanthomonas campestris pv. citri (ex Hasse 1915) Dye 1978 forms A, B/C/D, and E as X. smithii subsp. citri (ex Hasse) sp. nov. nom. rev. comb. nov., X. fuscans subsp. aurantifolii (ex Gabriel 1989) sp. nov. nom. rev. comb. nov., and X. alfalfae subsp. citrumelo (ex Riker and Jones) Gabriel et al., 1989 sp. nov. nom. rev. comb. nov.; X. campestris pv malvacearum (ex smith 1901) Dye 1978 as X. smithii subsp. smithii nov. comb. nov. nom. nov.; X. campestris pv. alfalfae (ex Riker and Jones, 1935) dye 1978 as X. alfalfae subsp. alfalfae (ex Riker et al., 1935) sp. nov. nom. rev.; and "var. fuscans" of X. campestris pv. phaseoli (ex Smith, 1987) Dye 1978 as X. fuscans subsp. fuscans sp. nov.Syst Appl Microbiol. 2005 Aug;28(6):494-518. doi: 10.1016/j.syapm.2005.03.017. Syst Appl Microbiol. 2005. PMID: 16104350
-
Resistance to citrus canker induced by a variant of Xanthomonas citri ssp. citri is associated with a hypersensitive cell death response involving autophagy-associated vacuolar processes.Mol Plant Pathol. 2017 Dec;18(9):1267-1281. doi: 10.1111/mpp.12489. Epub 2017 Jan 3. Mol Plant Pathol. 2017. PMID: 27647752 Free PMC article.
-
Citrus Canker Pathogen, Its Mechanism of Infection, Eradication, and Impacts.Plants (Basel). 2022 Dec 26;12(1):123. doi: 10.3390/plants12010123. Plants (Basel). 2022. PMID: 36616252 Free PMC article. Review.
-
Constructing a Novel Disease Resistance Mechanism Model for Cruciferous Crops: An Example From Black Rot.Mol Plant Pathol. 2025 Feb;26(2):e70060. doi: 10.1111/mpp.70060. Mol Plant Pathol. 2025. PMID: 39924905 Free PMC article. Review.
Cited by
-
Complete Genome Sequence Analysis of Ralstonia solanacearum Strain PeaFJ1 Provides Insights Into Its Strong Virulence in Peanut Plants.Front Microbiol. 2022 Feb 23;13:830900. doi: 10.3389/fmicb.2022.830900. eCollection 2022. Front Microbiol. 2022. PMID: 35273586 Free PMC article.
-
PthA4AT , a 7.5-repeats transcription activator-like (TAL) effector from Xanthomonas citri ssp. citri, triggers citrus canker resistance.Mol Plant Pathol. 2019 Oct;20(10):1394-1407. doi: 10.1111/mpp.12844. Epub 2019 Jul 5. Mol Plant Pathol. 2019. PMID: 31274237 Free PMC article.
-
A serralysin-like protein of Candidatus Liberibacter asiaticus modulates components of the bacterial extracellular matrix.Front Microbiol. 2022 Oct 19;13:1006962. doi: 10.3389/fmicb.2022.1006962. eCollection 2022. Front Microbiol. 2022. PMID: 36338045 Free PMC article.
-
Fusarium Species Associated with Diseases of Citrus: A Comprehensive Review.J Fungi (Basel). 2025 Mar 28;11(4):263. doi: 10.3390/jof11040263. J Fungi (Basel). 2025. PMID: 40278084 Free PMC article. Review.
-
Light- and Relative Humidity-Regulated Hypersensitive Cell Death and Plant Immunity in Chinese Cabbage Leaves by a Non-adapted Bacteria Xanthomonas campestris pv. vesicatoria.Plant Pathol J. 2024 Aug;40(4):358-376. doi: 10.5423/PPJ.OA.03.2024.0057. Epub 2024 Aug 1. Plant Pathol J. 2024. PMID: 39117335 Free PMC article.
References
-
- Adlung, N. , Prochaska, H. , Thieme, S. , Banik, A. , Bluher, D. , John, P. , Nagel, O. , Schulze, S. , Gantner, J. , Delker, C. , Stuttmann, J. and Bonas, U. (2016) Non‐host resistance induced by the Xanthomonas effector XopQ is widespread within the genus Nicotiana and functionally depends on EDS1. Front Plant Sci. 7, 1796. - PMC - PubMed
-
- An, C. , Wang, C. and Mou, Z. (2017) The Arabidopsis Elongator complex is required for nonhost resistance against the bacterial pathogens Xanthomonas citri subsp. citri and Pseudomonas syringae pv. phaseolicola NPS3121. New Phytol. 214, 1245–1259. - PubMed
-
- Arnaud, D. and Hwang, I. (2015) A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant, 8, 566–581. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

