Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity
- PMID: 30260752
- PMCID: PMC6397407
- DOI: 10.1152/japplphysiol.00719.2018
Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity
Abstract
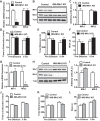
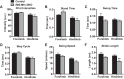
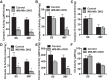
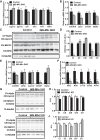
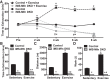
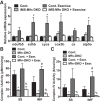
Endurance exercise has been shown to be a positive regulator of skeletal muscle metabolic function. Changes in mitochondrial dynamics (fusion and fission) have been shown to influence mitochondrial oxidative capacity. We therefore tested whether genetic disruption of mitofusins (Mfns) affected exercise performance in adult skeletal muscle. We generated adult-inducible skeletal muscle-specific Mfn1 (iMS-Mfn1KO), Mfn2 (iMS-Mfn2KO), and Mfn1/2 (iMS-MfnDKO) knockout mice. We assessed exercise capacity by performing a treadmill time to exhaustion stress test before deletion and up to 8 wk after deletion. Analysis of either the iMS-Mfn1KO or the iMS-Mfn2KO did not reveal an effect on exercise capacity. However, analysis of iMS-MfnDKO animals revealed a progressive reduction in exercise performance. We measured individual electron transport chain (ETC) complex activity and observed a reduction in ETC activity in both the subsarcolemmal and intermyofibrillar mitochondrial fractions specifically for NADH dehydrogenase (complex I) and cytochrome- c oxidase (complex IV), which was associated with a decrease in ETC subunit expression for these complexes. We also tested whether voluntary exercise training would prevent the decrease in exercise capacity observed in iMS-MfnDKO animals ( n = 10/group). However, after 8 wk of training we did not observe any improvement in exercise capacity or ETC subunit parameters in iMS-MfnDKO animals. These data suggest that the decrease in exercise capacity observed in the iMS-MfnDKO animals is in part the result of impaired ETC subunit expression and ETC complex activity. Taken together, these results provide strong evidence that mitochondrial fusion in adult skeletal muscle is important for exercise performance. NEW & NOTEWORTHY This study is the first to utilize an adult-inducible skeletal muscle-specific knockout model for Mitofusin (Mfn)1 and Mfn2 to assess exercise capacity. Our findings reveal a progressive decrease in exercise performance with Mfn1 and Mfn2 deletion. The decrease in exercise capacity was accompanied by impaired oxidative phosphorylation specifically for complex I and complex IV. Furthermore, voluntary exercise training was unable to rescue the impairment, suggesting that normal fusion is essential for exercise-induced mitochondrial adaptations.
Keywords: electron transport chain; exercise; mitochondria; mitofusin; skeletal muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Bannerman P, Burns T, Xu J, Miers L, Pleasure D. Mice hemizygous for a pathogenic mitofusin-2 allele exhibit hind limb/foot gait deficits and phenotypic perturbations in nerve and muscle. PLoS One 11: e0167573, 2016. [Erratum in PLoS One 13: e0204536, 2018.] doi: 10.1371/journal.pone.0167573. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

