Multiple origins and modularity in the spatiotemporal emergence of cerebellar astrocyte heterogeneity
- PMID: 30260948
- PMCID: PMC6178385
- DOI: 10.1371/journal.pbio.2005513
Multiple origins and modularity in the spatiotemporal emergence of cerebellar astrocyte heterogeneity
Abstract
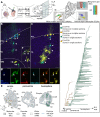
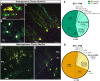
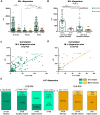
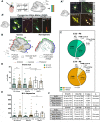
The morphological, molecular, and functional heterogeneity of astrocytes is under intense scrutiny, but how this diversity is ontogenetically achieved remains largely unknown. Here, by quantitative in vivo clonal analyses and proliferation studies, we demonstrate that the major cerebellar astrocyte types emerge according to an unprecedented and remarkably orderly developmental program comprising (i) a time-dependent decline in both clone size and progenitor multipotency, associated with clone allocation first to the hemispheres and then to the vermis(ii) distinctive clonal relationships among astrocyte types, revealing diverse lineage potentials of embryonic and postnatal progenitors; and (iii) stereotyped clone architectures and recurrent modularities that correlate to layer-specific dynamics of postnatal proliferation/differentiation. In silico simulations indicate that the sole presence of a unique multipotent progenitor at the source of the whole astrogliogenic program is unlikely and rather suggest the involvement of additional committed components.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures