Local NGF and GDNF levels modulate morphology and function of porcine DRG neurites, In Vitro
- PMID: 30260982
- PMCID: PMC6160011
- DOI: 10.1371/journal.pone.0203215
Local NGF and GDNF levels modulate morphology and function of porcine DRG neurites, In Vitro
Abstract
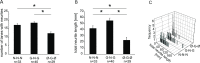
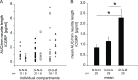
Nerve terminals of primary sensory neurons are influenced by their environment through target derived trophic factors, like nerve growth factor (NGF) or glial cell line-derived neurotrophic factor (GDNF). In mice, subpopulations of DRG neurons express receptors either for NGF or GDNF and therefore differentially respond to these neurotrophic factors. We probed neurite endings from porcine DRG neurons cultured in either NGF or GDNF and examined their shape, elongation and stimulus-evoked CGRP release. A compartmentalized culture system was employed allowing spatial separation of outgrown neurites from their somata and use of different growth factors in the compartments. We show that neurites of GDNF cultured somata extend into lateral compartments without added growth factor, unlike neurites of NGF cultured ones. Neurites of NGF cultured somata extend not only into NGF- but also into GDNF-containing compartments. GDNF at the site of terminals of NGF responsive somata led to a strong neurite arborization and formation of large growth cones, compared to neurites in medium with NGF. Functionally, we could detect evoked CGRP release from as few as 7 outgrown neurites per compartment and calculated release per mm neurite length. CGRP release was detected both in neurites from NGF and GDNF cultured somata, suggesting that also the latter ones are peptidergic in pig. When neurites of NGF cultured somata were grown in GDNF, capsaicin evoked a lower CGRP release than high potassium, compared to those grown in NGF. Our experiments demonstrate that the compartmented culture chamber can be a suitable model to assess neurite properties from trophic factor specific primary sensory neurons. With this model, insights into mechanisms of gain or loss of function of specific nociceptive neurites may be achieved.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Markus A, Patel TD, Snider WD (2002) Neurotrophic factors and axonal growth. Curr Opin Neurobiol 12: 523–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

