Characterization of a leukocidin identified in Staphylococcus pseudintermedius
- PMID: 30261001
- PMCID: PMC6160070
- DOI: 10.1371/journal.pone.0204450
Characterization of a leukocidin identified in Staphylococcus pseudintermedius
Abstract
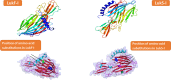
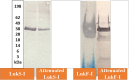
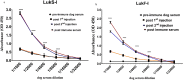
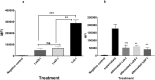
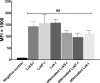
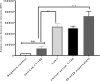
Bacterial infections from Staphylococcus pseudintermedius are the most common cause of skin infections (pyoderma) affecting dogs. Two component pore-forming leukocidins are a family of potent toxins secreted by staphylococci and consist of S (slow) and F (fast) components. They impair the innate immune system, the first line of defense against these pathogens. Seven different leukocidins have been characterized in Staphylococcus aureus, some of which are host and cell specific. Through genome sequencing and analysis of the S. pseudintermedius secretome using liquid chromatography mass spectrometry we identified two proteins, named "LukS-I" and "LukF-I", encoded on a degenerate prophage contained in the genome of S. pseudintermedius isolates. Phylogenetic analysis of LukS-I components in comparison to the rest of the leukocidin family showed that LukS-I was most closely related to S. intermedius LukS-I, S. aureus LukE and LukP, whereas LukF-I was most similar to S. intermedius LukF-I S. aureus gamma hemolysin subunit B. The killing effect of recombinant S. pseudintermedius LukS-I and LukF-I on canine polymorphonuclear leukocytes was determined using a flow cytometry cell permeability assay. The cytotoxic effect occurred only when the two recombinant proteins were combined. Engineered mutant versions of the two-component pore-forming leukocidins, produced through amino acids substitutions at selected points, were not cytotoxic. Anti-Luk-I produced in dogs against attenuated proteins reduced the cytotoxic effect of native canine leukotoxin which highlights the importance of Luk-I as a promising component in a vaccine against canine S. pseudintermedius infections.
Conflict of interest statement
We have the following interests. We have a patent application for PCT/US18/46281 "Staphylococcus pseudintermedius virulence factor compositions." This non-provisional application was filed by the University of Tennessee and is not associated with any transfer or sale of rights or ownership or any revenue. There are no further patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

